Generic Name : Acoltremon
Brand Name :Tryptyr
Drug Class : TRPM8 thermoreceptor agonist
Dosage : Ophthalmic Solution 0.003
Table of Contents
Introduction:
Dry eye disease (DED) affects millions of people worldwide, causing discomfort, blurred vision, and irritation that can impact daily life. Conventional treatments such as artificial tears or steroids often provide temporary relief. Enter acoltremone – a promising investigational drug that could redefine how we manage chronic dry eye symptoms.
In this comprehensive blog post, we explore what acoltremone is, how it works, its mechanism of action in dry eye disease, its benefits over existing treatments, clinical trial results, and frequently asked questions.
What is Tryptyr Ophthalmic Solution?
Acoltremone Ophthalmic Solution 0.003 is an investigational small molecule drug developed for the treatment of dry eye disease (DED), a condition characterized by inflammation and a lack of adequate tear production or quality. It is being evaluated for its anti-inflammatory and neuroprotective properties for the treatment of ocular surface disorders. Unlike traditional dry eye treatments that often provide symptomatic relief, acoltremone targets the underlying inflammatory and neurological pathways involved in tear regulation, making it a first-in-class therapeutic agent.
Understanding Dry Eye Disease (DED):
Dry Eye Disease, also known as keratoconjunctivitis sicca, is a multifactorial disease of the tear film and ocular surface. Key symptoms include:
- Grittiness or burning sensation
- Eye redness and irritation
- Sensitivity to light
- Blurred vision
- Fluctuating visual acuity
There are two main types of DED-
- Aqueous-deficient dry eye – due to reduced tear production
- Evaporative dry eye – due to rapid tear film evaporation
Inflammation and neurosensory dysfunction play a central role in both types. This is where acoltremon shows promise.
Mechanism of Action (MOA) in Dry Eye Disease :
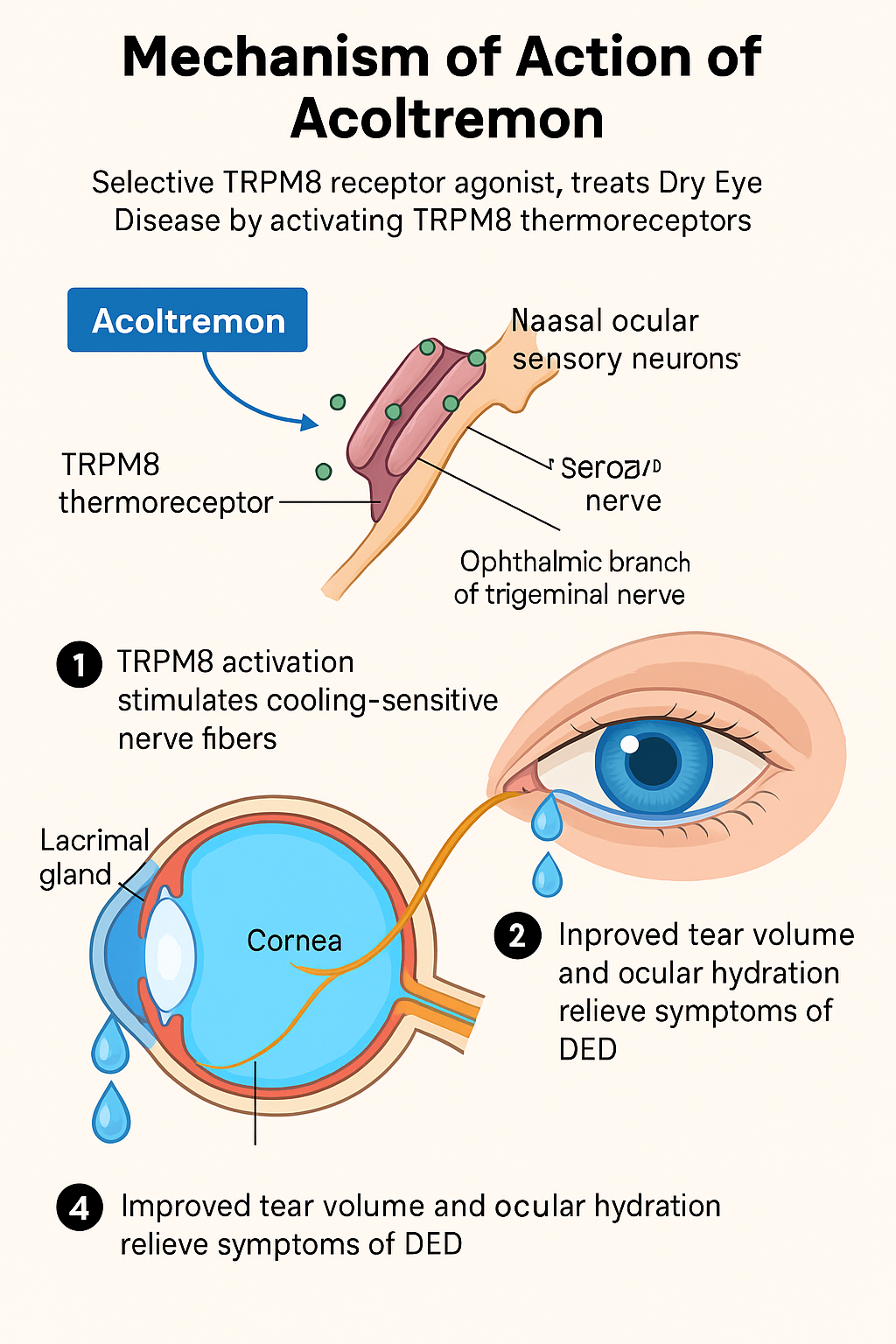
Acoltremon is a selective TRPM8 receptor agonist, designed to treat dry eye disease (DED) by activating cold-sensitive ion channels on the surface of the eye. Here’s how it works:
What is TRPM8?
TRPM8 (transient receptor potential melastatin 8) is a cold-sensitive ion channel found in sensory neurons, including neurons innervating the cornea and conjunctiva. These channels respond to cold temperatures and chemical agents such as menthol and control tear secretion and the blink reflex.
Acoltremon as a TRPM8 agonist
Activation of TRPM8:
Acoltremon selectively binds to TRPM8 receptors located in the ophthalmic branch of the trigeminal nerve, particularly in the nasal cavity and on the surface of the eye.
Stimulation of basal tear secretion:
By activating TRPM8, acoltremon increases basal (resting) tear production – which is often reduced in dry eye disease – without causing irritation or reflex tearing.
Cooling sensation and ocular surface hydration:
Activation of TRPM8 produces a mild cooling sensation, which soothes the ocular surface and helps restore ocular homeostasis, thereby reducing dryness, stiffness, and inflammation.
Anti-inflammatory response:
Acoltremone’s modulation of sensory nerve activity may also indirectly help suppress inflammatory pathways, providing long-term control of ocular surface inflammation.
Benefits of Opthalmic Solution for Dry Eye Disease :
Unlike artificial tears, steroids, or cyclosporine drops, acoltremon offers disease-modifying potential. Here’s why it stands out:
| Benefit | Acoltremon | Conventional Treatments |
|---|---|---|
| Targets inflammation | ✅ Yes | ⚠️ Partially |
| Stimulates nerve healing | ✅ Yes | ❌ No |
| Restores tear production | ✅ Yes | ⚠️ Limited |
| Reduces long-term symptoms | ✅ Yes | ⚠️ Temporary |
| Risk of side effects | ⚠️ Low | ⚠️ Moderate to high |
Clinical Trials and Research :
Encouraging results have been obtained from preclinical and early-stage clinical trials of acoltremone:
• Preclinical studies:
Significant improvements in corneal sensitivity, tear volume, and ocular surface healing were observed in animal models of dry eye.
• Phase I human trials:
Showed early signs of favorable safety, tolerability, and efficacy in patients with moderate to severe dry eye disease.
• Phase II trials:
Acoltremone is currently being evaluated for its long-term efficacy, dose optimization, and comparative performance with standard treatments.
Early results show that acoltremone can reduce symptoms and inflammation in just 2 to 4 weeks, making it one of the most promising treatments in development for DED.
• Phase III Clinical Trials: COMET‑2 & COMET‑3 :
Alcon conducted two pivotal randomized, double‑masked, vehicle‑controlled Phase III trials—COMET‑2 (N=465) and COMET‑3 (N=467)—evaluating Acoltremon 0.003% ophthalmic solution administered twice daily for 90 days, in patients with moderate to severe Dry Eye Disease.
Approval Status :
FDA Approval: May 29, 2025, for Acoltremon Ophthalmic Solution 0.003% (Tryptyr) for signs and symptoms of Dry Eye Disease. Yeasafili Biosimilar also lauched in june 2025 for DME treatment .
Expected Launch: Q3 2025 in the U.S
Dosage and Administration :
Based on early studies, it is being tested as:
- Ophthalmic solution 0.003 (eye drops)
- Once or twice daily application
- Under supervision of an eye care specialist
Final dosage instructions will depend on regulatory approval and further clinical evidence.
Potential Side Effects :
So far, acoltremon has shown a favorable safety profile, with most patients tolerating it well. Reported side effects (rare and mild) are:
- Slight stinging upon instillation
- Temporary blurred vision
- Mild redness of the eyes
- No systemic side effects have been observed
Importantly, no serious ocular complications have been reported in early-stage trials.
Who Should Consider this Opthalmic solution ?
Opthalmic solution may be beneficial for patients who:
- Have moderate to severe dry eye disease
- Do not respond well to artificial tears or steroids
- Have dry eye associated with nerve damage
- Experience neuropathic eye pain
- Want a treatment with disease-modifying potential.
As always, consult a licensed ophthalmologist or ophthalmologist before starting any treatment.
Conclusion :
Dry eye disease is not just an annoying condition – for many, it is a chronic and disabling condition. Acoltremon offers hope as a next-generation therapy that not only treats symptoms but also addresses the underlying causes of the disease.
With its multi-action mechanism of action, early positive trial results, and minimal side effects, acoltremon could soon become a great solution for millions of people suffering from dry eyes. Although it is still in development, the future looks bright for the drug and the patients who need it.
Stay tuned for updates on acoltremone’s approval status, clinical results, and how it could soon change the landscape of eye care.
FAQs :
Q1: What is Tryptyr used for?
Q2: How does Acoltremon help with dry eyes?
Q3: Is Acoltremon(Tryptyr) FDA approved?
Q4: Can Acoltremon replace artificial tears?
Q5: Who manufactures Tryptyr?
Disclaimer: This blog post is for informational purposes only and does not constitute medical advice. Always consult a qualified healthcare professional before making any medical decisions.

2 thoughts on “Acoltremon Ophthalmic Solution 0.003 for Dry Eye Disease: A New Breakthrough in Ocular Therapy”