Yesafili biosimilar is game changer who have problem in their vision .
Generic Name: Aflibercept
Class: Anti-angiogenic ophthalmic agents
Form : single-dose vial for ophthalmic injection
Table of Contents
What is Yesafili Biosimiliar ?
- Yesafili is a biosimilar of Aflibercept (Eylea), a well-known VEGF inhibitor(Vascular Endothelial Growth Factor inhibitor) used in the treatment of serious eye conditions like wet age-related macular degeneration (nAMD), diabetic macular edema (DME), and retinal vein occlusion (RVO).
- As a biosimilar, Yesafili closely matches the reference drug in terms of safety, efficacy, and quality, offering a more affordable alternative for patients needing long-term retinal disease management.
- The Yesafili (aflibercept) , developed by Biocon Biologics, launch in Canada on July 4, 2025. This follows Health Canada’s approval of the biosimilar on June 26, 2025. In the US, the launch is planned for the second half of 2026, or earlier under certain circumstances, or earlier in some cases.
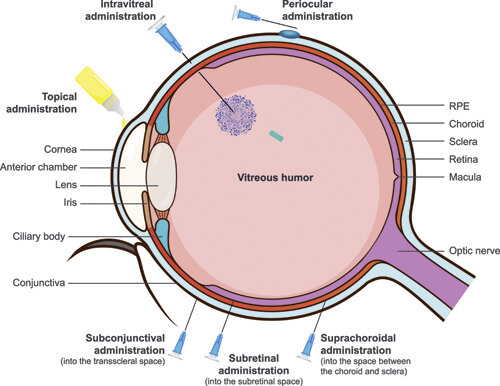
How Does Yesafili Biosimilar Differ from Eylea?
- Yesafili is a biosimilar of Eylea, meaning it has the same therapeutic effect, quality, and safety profile.
- Both are used to treat retinal diseases like wet AMD, diabetic macular edema, and retinal vein occlusion.
- Its offers a more affordable option, especially in public healthcare settings.
- Eylea has more global regulatory approvals, but Yesafili is expected to expand access where biosimilars are accepted.
| Feature | Yesafili Biosimilar | Eylea (Reference Drug) |
|---|---|---|
| Type | Biosimilar | Biologic (Original) |
| Manufacturer | Coherus / Bioeq / Formycon | Regeneron / Bayer |
| Approval Year | 2024 (Canada, EU) | 2011 (USA) |
| Active Ingredient | Aflibercept | Aflibercept |
| Cost | Lower | Higher |
| Availability | Limited (2024–25 rollout) | Global |
| Clinical Trial Scope | Biosimilarity studies | Full Phase I–III trials |
| Patent Status | Post-patent launch | Patent expiry ongoing |
| Indications | Similar | Similar |
| Trust & Usage | Growing | Established |
How Does Yesafili works ?
1.VEGF Inhibition :
Yesafili targets and binds to Vascular Endothelial Growth Factor-A (VEGF-A) — a protein that promotes the growth of abnormal blood vessels in the retina.
2.Prevents Leakage :
By blocking VEGF-A, Yesafili prevents the formation of leaky and fragile blood vessels, which can damage the retina and impair vision.
3.Reduces Fluid Build-Up :
It helps in reducing retinal swelling and fluid accumulation, especially in conditions like wet AMD and diabetic macular edema.
4.Stabilizes or Improves Vision :
Clinical studies show that it can maintain or even improve vision in patients with retinal vascular diseases.
5.Mimics Aflibercept (Eylea) By Yesafili Biosimilar :
Yesafili works in the same way as Aflibercept, offering similar therapeutic effects at a lower cost.
Uses of The Yesafili Biosimilar :
1. Neovascular (Wet) Age-Related Macular Degeneration (nAMD):
Yesafili is used to treat wet AMD, a condition where abnormal blood vessels grow under the retina, causing bleeding and fluid leakage that leads to central vision loss.
2. Diabetic Macular Edema (DME):
It helps manage macular swelling caused by diabetes, which can blur or distort vision due to fluid leakage from damaged retinal blood vessels.
3. Macular Edema following Retinal Vein Occlusion (RVO):
Yesafili is indicated for treating macular swelling resulting from blocked retinal veins, which restrict normal blood flow in the retina.4. Myopic Choroidal Neovascularization (mCNV):
It is also used in highly myopic (near-sighted) patients who develop abnormal blood vessels beneath the retina, threatening vision clarity.
Yesafili Dosage :
- Dose: 2 mg (0.05 mL) injected into the affected eye
- Schedule:
- Every 4 weeks for the initial 3 months
- Then once every 8–12 weeks depending on response
The injection is performed by an ophthalmologist in a clinical setting under sterile conditions.
Administration of Yesafili Biosimilar :
- Yesafili is administered by intravitreal injection, meaning it is injected directly into the vitreous cavity of the eye.
- The injection must be performed by a qualified ophthalmologist or retina specialist in a sterile clinical environment.
- The procedure is done under strict aseptic (germ-free) conditions to minimize the risk of infection, such as endophthalmitis.
- The procedure is done under strict aseptic (germ-free) conditions to minimize the risk of infection, such as endophthalmitis.
Side Effects of Yesafili :
1.Common Side Effects of Yesafili
These are usually mild and temporary:
- Eye pain or discomfort after injection
- Blurry vision
- Increased eye pressure (temporary)
- Redness in the eye
- Floaters (black spots or shadows in vision)
- Watery eyes or dryness
2.Serious Side Effects Yesafili Biosimilar
Though rare, these require immediate medical attention:
- Endophthalmitis – a serious eye infection causing pain, redness, and vision loss
- Retinal detachment – separation of the retina from its normal position
- Intraocular inflammation – swelling inside the eye
- Cataract formation (with repeated injections)
- Vision loss (very rare)
3.Allergic Reactions (Rare)
- Swelling of the eyelids
- Itching or rash around the eyes
- Hypersensitivity to the drug
If you experience sudden eye pain, severe vision changes, increased sensitivity to light, or signs of infection after an injection, contact your eye doctor immediately.
Storage Conditions for Yesafili Biosimilar :
| Parameter | Condition |
| Storage Temperature | 2°C to 8°C (Refrigerated) |
| Freezing | ❌ Do not freeze |
| Light Protection | Store in original carton to protect from light |
| Shaking | ❌ Do not shake |
| Appearance Check | Solution should be clear to slightly yellow, no particles |
| After Opening | Use immediately after opening vial or syringe |
| Post-Puncture Storage | ❌ Do not store after puncturing – discard unused portion |
| Transport Guidelines | Maintain cold chain (2–8°C) during transportation |
Precautions for Using Yesafili :
- Avoid Use in Eye Infections:
Do not use Yesafili if there is an active or suspected ocular or periocular infection (e.g., conjunctivitis, blepharitis, or endophthalmitis). - Use with Sterile Technique:
The injection must be administered under strict aseptic (sterile) conditions to avoid serious complications like endophthalmitis. - Monitor Intraocular Pressure:
Patients should be monitored for increased intraocular pressure (IOP) immediately after injection. - Inflammation Risk:
Watch for signs of intraocular inflammation such as eye pain, redness, or blurred vision following the injection. - Pregnancy and Breastfeeding:
Use with caution in pregnant or breastfeeding women. There is limited data on the safety in these groups. - Hypersensitivity Reactions:
Patients with known hypersensitivity to aflibercept or similar biologics should not receive Yesafili. - Bilateral Use:
If both eyes need treatment, each injection should be prepared and administered separately to prevent cross-contamination. - Immunocompromised Patients:
Caution is advised in patients with compromised immune systems, as the risk of infection may be higher.
Conclusion :
Yesafili is a promising biosimilar to Aflibercept, offering effective and affordable treatment for serious retinal diseases like wet age-related macular degeneration, diabetic macular edema, and retinal vein occlusion. With a similar safety and efficacy profile to the reference drug, Yesafili expands access to advanced eye care without compromising on quality. As it becomes more widely available, it stands to make a significant impact in ophthalmic healthcare—especially for patients requiring long-term therapy. Always consult a retina specialist before starting or switching to any intravitreal therapy.
FAQ For Yesafili Biosimilar:
Q1: What is Yesafili biosimilar?
Q2: Is Yesafili as effective as Eylea?
Q3: Why is Yesafili cheaper than Eylea?
Q4: Is Yesafili available in India or the US?
Disclaimer: This blog post is for informational purposes only and does not constitute medical advice. Always consult a qualified healthcare professional before making any medical decisions.

1 thought on ““Yesafili Biosimilar Revealed: A Cost-Effective & Trusted Option for Vision Care””