Table of Contents
Introduction
The approval of novel therapies for rare pediatric diseases represents one of the most critical challenges in modern medicine. Many rare genetic disorders remain underdiagnosed, undertreated, and historically overlooked due to limited commercial incentives and scientific complexity. Against this backdrop, the Zycubo FDA approval stands as a landmark achievement in pediatric rare disease therapy.
On January 12, 2026, the U.S. Food and Drug Administration granted Zycubo FDA approval as the first and only FDA-approved treatment for Menkes disease, a rare and often fatal neurodegenerative disorder that primarily affects infants and young children. Zycubo, a subcutaneous copper histidinate injection, fundamentally changes the therapeutic landscape for this condition by addressing its underlying biological cause rather than merely managing symptoms.
The Zycubo FDA approval is particularly significant because Menkes disease, if left untreated, leads to severe neurological deterioration and early mortality. By enabling effective and regulated copper replacement therapy during early development, Zycubo offers a life-saving intervention where none previously existed.
What Is Zycubo?
Zycubo is a pharmaceutical formulation of copper histidinate, specifically designed for subcutaneous administration in pediatric patients diagnosed with Menkes disease. Following the Zycubo FDA approval, it became the first standardized, FDA-regulated copper replacement therapy approved for this rare condition.
Unlike historical copper supplementation approaches, Zycubo is:
- Manufactured under strict pharmaceutical quality standards
- Clinically evaluated for safety and efficacy
- Approved with defined dosing and monitoring guidelines
The Zycubo ensures consistent copper delivery, predictable pharmacological behavior, and improved patient outcomes when administered early in life.
Understanding Menkes Disease
Menkes disease is a rare X-linked genetic disorder caused by mutations that impair copper transport within the body. Copper is an essential trace element required for numerous enzymatic reactions, particularly those involved in neurological development, connective tissue formation, and energy metabolism.
Pathophysiology of Menkes Disease
In Menkes disease:
- Copper absorption and distribution are severely impaired
- Copper fails to reach the brain and other vital organs
- Copper-dependent enzymes become dysfunctional
This leads to:
- Progressive neurodegeneration
- Developmental delay
- Hypotonia
- Seizures
- Failure to thrive
- Early childhood death
Before the Zycubo , treatment options were inconsistent and largely experimental, with no FDA-approved therapy available.
Unmet Medical Need Before Zycubo FDA Approval
Prior to the Zycubo FDA approval, families and clinicians faced significant challenges:
- Lack of standardized treatment protocols
- Use of unapproved copper formulations
- Variable clinical outcomes
- Limited survival benefit
Early diagnosis alone was insufficient without a reliable therapeutic option. The Zycubo FDA approval directly addresses this unmet need by providing a validated, disease-modifying treatment.
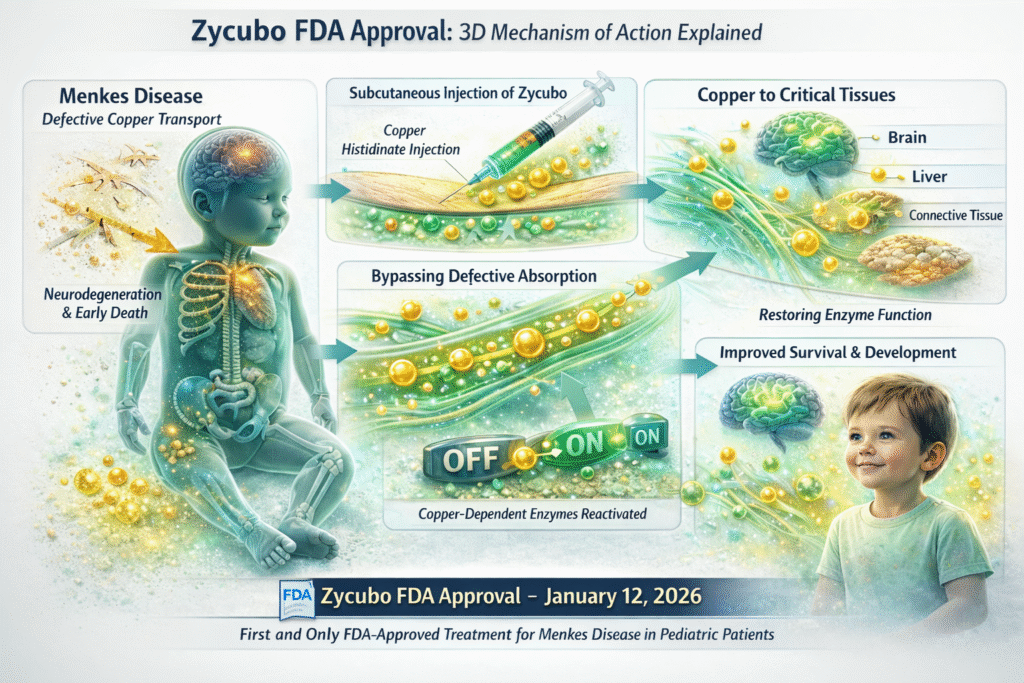
Mechanism of Action (MOA) of Zycubo
The mechanism of action was a cornerstone of the Zycubo FDA approval, as the therapy directly targets the root cause of Menkes disease.
How Zycubo Works
Zycubo delivers copper histidinate, a bioavailable copper complex that can bypass defective intestinal copper transport mechanisms.
Detailed MOA Breakdown
- Subcutaneous Injection
Zycubo is administered subcutaneously, allowing controlled systemic absorption. - Copper Distribution
The copper histidinate complex enters circulation and distributes to copper-deficient tissues. - Cellular Uptake
Copper reaches the central nervous system and peripheral organs. - Enzymatic Restoration
Copper-dependent enzymes regain functionality. - Neurodevelopmental Support
Early restoration supports brain development and prevents irreversible damage.
This precise biological correction was a major factor in achieving the Zycubo FDA approval.
Clinical Evidence Supporting Zycubo FDA Approval
Clinical evaluation demonstrated that early initiation of Zycubo therapy:
- Significantly improves survival rates
- Delays or prevents neurological deterioration
- Enhances developmental milestones
- Improves overall quality of life
These outcomes distinguish Zycubo from historical copper supplementation strategies and underpin the Zycubo .
Zycubo FDA Approval: Regulatory Overview
The Zycubo FDA approval was officially granted on January 12, 2026.
Approval Highlights
- Drug Name: Zycubo
- Active Ingredient: Copper histidinate
- Indication: Menkes disease
- Patient Population: Pediatric patients
- Route of Administration: Subcutaneous injection
- Approval Status: First and only FDA-approved treatment
The approval acknowledges the profound unmet medical need and the therapy’s favorable benefit-risk profile.
Dosage and Administration
According to the Zycubo FDA approval, dosing is:
- Initiated early in infancy whenever possible
- Adjusted based on body weight and copper levels
- Administered under specialist supervision
Consistent administration and monitoring are essential for optimal outcomes.
Side Effects of Zycubo
Safety assessment was central to the Zycubo .
Common Side Effects
- Injection-site reactions
- Mild irritability
- Transient gastrointestinal discomfort
Less Common Side Effects
- Temporary elevations in copper levels
- Mild laboratory abnormalities
Serious Adverse Events
Serious adverse effects were rare when dosing and monitoring guidelines were followed.
Safety Monitoring and Risk Management
Post-approval safety strategies associated with the Zycubo FDA approval include:
- Regular copper level monitoring
- Neurological assessments
- Growth and developmental evaluations
These measures ensure long-term safety and sustained therapeutic benefit.
Drug Interaction Profile
Zycubo has limited interaction potential, but caution is advised with:
- Other copper-containing supplements
- Drugs affecting metal metabolism
Medication reconciliation is recommended following the Zycubo FDA approval.
Use in Special Populations
The Zycubo FDA approval specifically applies to pediatric patients. Use outside approved populations should be guided by clinical judgment and regulatory standards.
Impact of Zycubo FDA Approval on Pediatric Rare Diseases
The Zycubo FDA approval represents:
- A paradigm shift from palliative care to disease modification
- Validation of early genetic screening programs
- Increased momentum for orphan drug development
It serves as a model for future pediatric rare disease approvals.
Future Directions After Zycubo FDA Approval
Ongoing efforts focus on:
- Earlier diagnosis through newborn screening
- Long-term neurodevelopmental outcome studies
- Optimized dosing strategies
The Zycubo FDA approval is expected to influence treatment guidelines globally.
Conclusion
The Zycubo FDA approval marks a historic breakthrough in pediatric medicine. As the first and only FDA-approved treatment for Menkes disease, Zycubo offers a life-saving, disease-modifying therapy that directly addresses copper deficiency at its biological source.
This approval redefines expectations for survival and neurological outcomes, offering renewed hope to families affected by this devastating disorder.

