Table of Contents
Biologic therapies have revolutionized the treatment of chronic inflammatory diseases over the last two decades. Among these, ustekinumab has stood out as a highly effective monoclonal antibody targeting immune-mediated disorders. With the growing interest in affordability and access, ustekinumab biosimilar FDA approval has become a highly discussed topic in the medical and pharmaceutical community.
Understanding the implications of ustekinumab FDA approval requires exploring not just regulatory aspects, but also clinical efficacy, mechanism of action (MOA), safety profile, drug interactions, cost impact, and patient outcomes. Additionally, some discussions have emerged regarding its potential relevance to hypertrophic cardiomyopathy (HCM), though this requires clarification.
This comprehensive guide provides deep insight into all these areas.
Introduction:
The healthcare system constantly seeks ways to improve patient access to life-changing therapies while controlling rising costs. Biologic medications, although highly effective, are expensive due to their complex manufacturing processes. The introduction of biosimilars is a strategic solution.
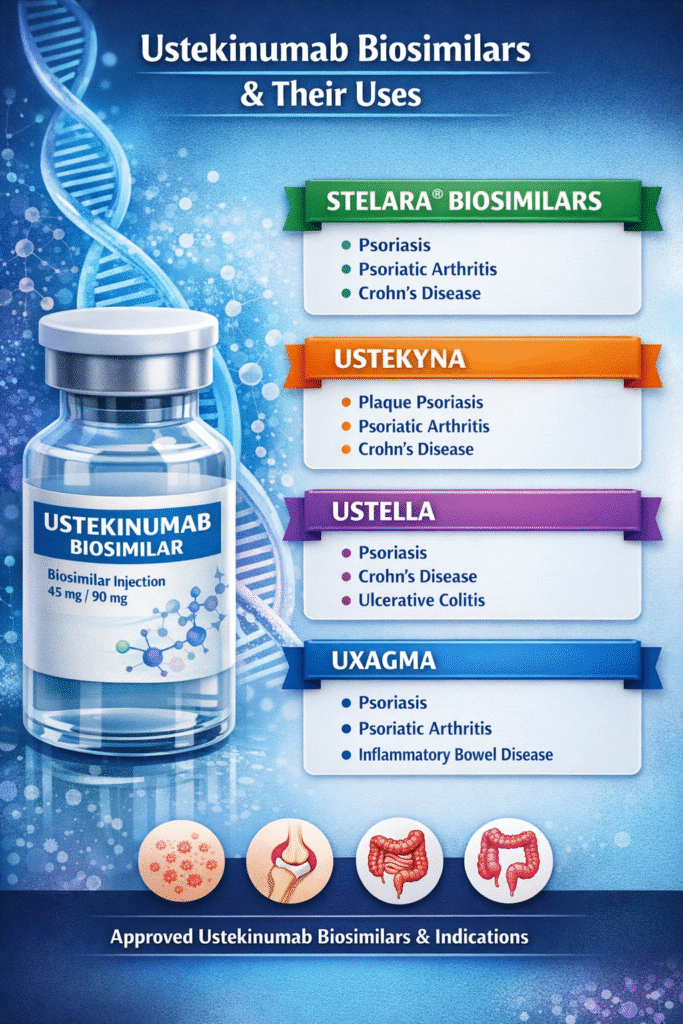
Ustekinumab biosimilar FDA approval represents a significant milestone in expanding access to biologic therapy for autoimmune conditions such as:
- Plaque psoriasis
- Psoriatic arthritis
- Crohn’s disease
- Ulcerative colitis
When discussing ustekinumab biosimilar FDA approval, it is important to understand that biosimilars are not generic drugs. Instead, they are highly similar biological products that match the original drug in terms of safety, purity, and potency.
The approval pathway for biosimilars is stringent, ensuring patient safety and clinical equivalence.
Understanding Ustekinumab: The Reference Biologic
Before analyzing ustekinumab biosimilar FDA approval, it is essential to understand what ustekinumab is.
Ustekinumab is a human monoclonal antibody designed to target inflammatory cytokines involved in autoimmune disorders. It specifically binds to the p40 subunit shared by:
- Interleukin-12 (IL-12)
- Interleukin-23 (IL-23)
These cytokines play a central role in immune system activation and inflammatory signaling pathways.
The introduction of ustekinumab biosimilar FDA approval ensures that biosimilar products mimic this complex biological function precisely.
Mechanism of Action (MOA)
Targeting IL-12 and IL-23 Pathways
The primary mechanism behind ustekinumab involves selective inhibition of IL-12 and IL-23. These cytokines activate T-helper cells (Th1 and Th17), which promote inflammation.
Through targeted binding:
- Ustekinumab blocks cytokine signaling
- Reduces inflammatory response
- Decreases immune-mediated tissue damage
The scientific basis of ustekinumab biosimilar FDA approval ensures that biosimilars replicate this mechanism with no clinically meaningful difference.
Immunological Cascade Modulation
Inflammatory diseases are driven by overactivation of immune pathways. By interrupting cytokine signaling:
- T-cell activation decreases
- Inflammatory mediators reduce
- Tissue inflammation subsides
Every product receiving ustekinumab biosimilar FDA approval must demonstrate identical immune pathway inhibition through advanced analytical and clinical studies.
FDA Approval Process for Biosimilars
The process leading to ustekinumab biosimilar FDA approval is one of the most rigorous regulatory pathways in modern pharmacology.
Step 1: Analytical Similarity Testing
Scientists conduct detailed molecular comparisons including:
- Protein structure
- Binding affinity
- Biological activity
Step 2: Nonclinical Studies
Animal studies evaluate toxicity and pharmacologic behavior.
Step 3: Clinical Trials
Human trials compare:
- Pharmacokinetics (PK)
- Pharmacodynamics (PD)
- Immunogenicity
- Safety
- Efficacy
Only after meeting strict equivalence criteria does the FDA grant ustekinumab biosimilar FDA approval.
Clinical Trial Evidence Supporting Ustekinumab Biosimilar FDA Approval
Clinical trials are central to ustekinumab biosimilar FDA approval.
Studies typically assess:
- Psoriasis Area Severity Index (PASI) response
- Clinical remission rates in Crohn’s disease
- Safety endpoints
- Immunogenic antibody formation
Results consistently demonstrate:
- Equivalent therapeutic response
- Comparable safety profiles
- No new adverse effects
This robust data forms the foundation of ustekinumab biosimilar FDA approval.
Side Effects and Safety Profile
Safety remains paramount even after ustekinumab biosimilar FDA approval.
Common Side Effects
- Upper respiratory infections
- Headache
- Injection site reactions
- Fatigue
- Nasopharyngitis
These side effects mirror the reference biologic.
Serious But Rare Adverse Events
- Serious infections
- Hypersensitivity reactions
- Rare malignancy concerns
- Reversible posterior leukoencephalopathy syndrome (extremely rare)
The rigorous process behind ustekinumab biosimilar FDA approval ensures these risks are not elevated compared to the original drug.
Drug Interactions
Understanding drug interactions is essential when considering therapy after ustekinumab biosimilar FDA approval.
Immunosuppressive Drugs
Concurrent use may increase infection risk.
Live Vaccines
Live vaccines should generally be avoided.
Other Biologic Agents
Combining biologics may increase immune suppression.
Healthcare providers carefully evaluate medication lists before initiating therapy following ustekinumab biosimilar FDA approval.
Cost Benefits and Healthcare Economics
One of the strongest arguments supporting ustekinumab biosimilar FDA approval is economic impact.
Reduced Cost
Biosimilars typically cost 15–35% less than reference biologics.
Insurance Coverage Expansion
Payers often encourage biosimilar use due to cost-effectiveness.
Global Accessibility
Lower prices increase patient access worldwide.
The broader economic benefits highlight the importance of ustekinumab biosimilar FDA approval in sustainable healthcare systems.
Ustekinumab Biosimilar FDA Approval and Hypertrophic Cardiomyopathy (HCM)
A key clarification:
There is currently no clinical evidence supporting the use of ustekinumab or any product under ustekinumab biosimilar FDA approval for hypertrophic cardiomyopathy.
HCM is a genetic cardiac condition involving:
- Abnormal thickening of the heart muscle
- Diastolic dysfunction
- Risk of arrhythmias
Standard treatments include:
- Beta-blockers
- Calcium channel blockers
- Myosin inhibitors
- Septal reduction procedures
While inflammation research continues in cardiovascular medicine, ustekinumab biosimilar FDA approval does not currently extend to HCM treatment.
Switching from Originator to Biosimilar
Many patients ask whether switching is safe after ustekinumab biosimilar FDA approval.
Studies show:
- No loss of efficacy
- No increased immunogenicity
- No new safety concerns
Physicians evaluate:
- Disease stability
- Insurance coverage
- Patient comfort
Switching decisions should always involve shared decision-making.
Immunogenicity Considerations
Immunogenicity refers to the body forming antibodies against the drug.
During the evaluation leading to ustekinumab biosimilar , immunogenicity rates must match the reference product.
Clinical evidence shows:
- Similar anti-drug antibody rates
- No increase in neutralizing antibodies
This reassures clinicians about long-term safety.
Pharmacokinetics and Pharmacodynamics
Products receiving FDA approval must demonstrate:
- Similar absorption
- Comparable distribution
- Equivalent metabolism
- Matching elimination profiles
PK/PD equivalence ensures identical dosing schedules.
Post-Marketing Surveillance
Even after ustekinumab biosimilar FDA approval, pharmacovigilance continues.
Real-World Data Collection
- Safety registries
- Electronic health records
- Adverse event reporting systems
This continuous monitoring safeguards patient safety long-term.
Future Outlook of Biosimilars
The success of ustekinumab biosimilar FDA approval signals a broader transformation in biologic medicine.
Future trends include:
- Increased competition
- Greater affordability
- Expanded biosimilar pipelines
- Improved healthcare equity
The long-term impact of ustekinumab biosimilar FDA approval extends beyond a single drug—it reshapes biologic therapy accessibility globally.
Conclusion
The emergence of ustekinumab biosimilar FDA approval marks a transformative shift in biologic therapy. By maintaining strict regulatory standards, ensuring equivalent clinical outcomes, and promoting cost savings, biosimilars represent the future of accessible medicine.
While it is not indicated for hypertrophic cardiomyopathy, the broader impact of ustekinumab biosimilar FDA approval on inflammatory disease management is substantial. Patients, clinicians, and healthcare systems all benefit from increased access, affordability, and therapeutic choice.
As the biosimilar landscape continues evolving, ustekinumab biosimilar FDA approval stands as a powerful example of innovation meeting regulation to improve global healthcare outcomes.

