Table of Contents
Introduction
The rapid advancement of biologic therapies has transformed the treatment landscape for autoimmune and antibody-mediated diseases. Among these innovations, Uplizna (inebilizumab-cdon) stands out as a targeted monoclonal antibody designed to modify disease progression rather than merely suppress symptoms. Understanding the Uplizna Mechanism of Action is essential for clinicians, pharmacists, researchers, and patients seeking deeper insight into how this therapy controls immune-driven diseases.
The Uplizna Mechanism of Action focuses on selective depletion of pathogenic B cells that play a critical role in autoantibody production. By intervening at an upstream point in the immune cascade, Uplizna offers sustained disease control in conditions where traditional immunosuppressive therapies often fall short.
This comprehensive article provides an in-depth explanation of the Uplizna Mechanism of Action, along with its pharmacological design, clinical benefits, FDA approval journey, side effects, drug interactions, dosing considerations, and long-term monitoring requirements.
What Is Uplizna?
Uplizna is a humanized monoclonal antibody specifically engineered to target CD19, a protein expressed on the surface of B cells throughout multiple stages of development. These B cells are responsible for producing antibodies, including pathogenic autoantibodies that drive autoimmune diseases.
Unlike conventional immunosuppressants that broadly dampen immune activity, the Uplizna Mechanism allows for precise immune modulation. This targeted approach reduces disease activity while preserving essential immune functions as much as possible.
Understanding the Uplizna Mechanism of Action
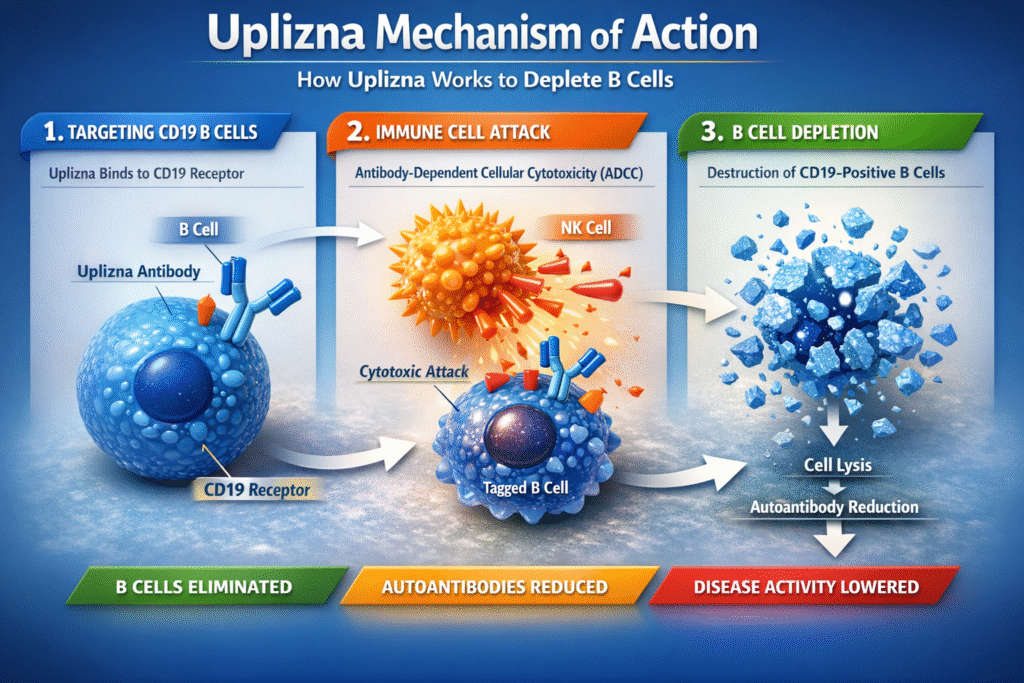
Overview of the Mechanism of Action
The Uplizna Mechanism of Action is centered on CD19-directed B-cell depletion. CD19 is expressed across a wide range of B-cell subsets, including:
- Pre-B cells
- Mature naïve B cells
- Memory B cells
- Plasmablasts
By targeting CD19, the Uplizna Mechanism of Action achieves broader B-cell depletion than therapies that target CD20 alone. This distinction is critical because plasmablasts and early plasma cells can continue producing autoantibodies even when CD20-positive cells are eliminated.
CD19 Binding and Immune Cell Targeting
After intravenous administration, Uplizna binds with high specificity to the CD19 antigen on B cells. This binding activates immune effector mechanisms that lead to the destruction of these cells.
A key component of the Mechanism of Action is antibody-dependent cellular cytotoxicity (ADCC). In this process:
- Uplizna binds to CD19 on B cells
- Immune effector cells recognize the Fc region of Uplizna
- Targeted B cells are destroyed
This precise immune targeting explains the potent and sustained effects observed with the Uplizna Mechanism of Action.
Glycoengineering and Enhanced ADCC
A unique feature of the Uplizna Mechanism is its glycoengineered Fc region. The antibody has been modified to enhance its interaction with immune effector cells, particularly natural killer (NK) cells.
This enhanced Fc-receptor binding significantly improves ADCC efficiency, allowing Uplizna to eliminate pathogenic B cells more effectively. As a result, the Uplizna Mechanism of Action delivers robust immune modulation with a predictable safety profile.
Reduction of Autoantibody Production
Autoantibodies are central drivers of tissue damage in many autoimmune disorders. The Uplizna Mechanism of Action reduces autoantibody levels by:
- Depleting B cells that differentiate into antibody-secreting cells
- Preventing formation of new plasmablasts
- Limiting immune memory related to disease activity
This upstream suppression of autoantibody production is a defining advantage of the Uplizna Mechanism of Action.
Diseases Treated Through the Uplizna Mechanism of Action
Neuromyelitis Optica Spectrum Disorder (NMOSD)
NMOSD is a severe autoimmune disease affecting the optic nerves and spinal cord. Pathogenic autoantibodies drive inflammation and neurological damage.
By suppressing the B cells responsible for these antibodies, the Uplizna Mechanism of Action significantly reduces relapse rates and long-term disability in NMOSD patients.
Immunoglobulin G4-Related Disease (IgG4-RD)
IgG4-RD is characterized by immune-mediated fibrosis affecting multiple organs. B-cell dysfunction plays a central role in disease progression.
The Uplizna Mechanism of Action interrupts this process by eliminating CD19-positive cells, offering sustained disease control and reduced organ damage.
Generalized Myasthenia Gravis (gMG)
In generalized myasthenia gravis, autoantibodies impair neuromuscular transmission, leading to muscle weakness and fatigue.
The Uplizna Mechanism of Action reduces antibody production, resulting in improved muscle strength, reduced symptom severity, and enhanced quality of life.
FDA Approval and Regulatory Milestones
Uplizna received FDA approval following extensive clinical trials demonstrating:
- Significant reduction in disease relapse
- Improved functional outcomes
- Durable B-cell depletion
- Acceptable safety profile
The FDA approval validated the clinical importance of the Uplizna Mechanism of Action and established it as a disease-modifying therapy rather than a symptomatic treatment.
Dosage and Administration
Uplizna is administered via intravenous infusion. Standard treatment involves:
- Initial loading doses
- Maintenance dosing every six months
This infrequent dosing schedule is a practical advantage of the Uplizna Mechanism of Action, reducing treatment burden and improving adherence.
Side Effects of Uplizna
Common Side Effects
Common side effects associated with the Uplizna Mechanism of Action include:
- Upper respiratory infections
- Urinary tract infections
- Headache
- Joint pain
- Mild infusion-related reactions
These effects are consistent with targeted immune modulation.
Serious Side Effects
Less common but clinically significant risks include:
- Increased susceptibility to infections
- Reactivation of latent viral infections
- Reduced immunoglobulin levels with prolonged therapy
Proper patient selection and monitoring are essential when using therapies based on the Uplizna Mechanism of Action.
Drug Interactions
Immunosuppressive Medications
Combining Uplizna with other immunosuppressive agents may increase infection risk. Clinical judgment is required when layering therapies that act through immune suppression.
Vaccines
Live vaccines are generally avoided during treatment because the Uplizna Mechanism of Action reduces immune responsiveness.
Monitoring and Long-Term Safety
Long-term management of patients receiving Uplizna includes:
- Monitoring infection signs
- Periodic immunoglobulin assessment
- Evaluation of B-cell recovery
These measures ensure safe and effective use of therapies based on the Uplizna Mechanism of Action.
Advantages of the Uplizna Mechanism of Action
Key advantages include:
- Targeted immune modulation
- Broad B-cell depletion via CD19 targeting
- Durable disease control
- Reduced relapse rates
- Infrequent dosing schedule
These features make the Uplizna Mechanism of Action a major advancement in autoimmune disease therapy.
Conclusion
The Uplizna Mechanism of Action represents a significant step forward in targeted immunotherapy. By selectively depleting CD19-positive B cells, Uplizna addresses the root cause of antibody-mediated autoimmune diseases rather than simply controlling symptoms. Its FDA approval across multiple serious conditions underscores the clinical importance of this innovative mechanism.
With its precision targeting, durable efficacy, and manageable safety profile, the Uplizna Mechanism of Action continues to redefine standards of care in autoimmune disease management.

