Table of Contents
- Generic Name: Taletractinib
- Brand Name: Ibtrozi
- Dosage : Tablet
Introduction :
Lung cancer is one of the most challenging cancers to treat globally. Of its various forms, non-small cell lung cancer (NSCLC) is the most common, with many patients harboring specific genetic alterations such as ROS1 or NTRK fusions. This has opened the door to targeted therapies, and talatrectinib has emerged as a promising treatment in this field.
In this article, we will explore everything you need to know about talatrectinib – its mechanism of action, approved uses, clinical trial results, side effects, and more.
What is Taletrectinib?
Taletractinib (development code: AB-106 or DS-6051b) is a next-generation ROS1 and pan-NTRK tyrosine kinase inhibitor (TKI). It is currently being developed for the treatment of patients with advanced solid tumors, particularly NSCLC with ROS1 rearrangements or NTRK gene fusions.
What makes taletractinib unique is its ability to target ROS1 and NTRK fusion-positive cancers—particularly patients who have developed resistance to first-generation TKIs such as crizotinib.
Mechanism of Action :
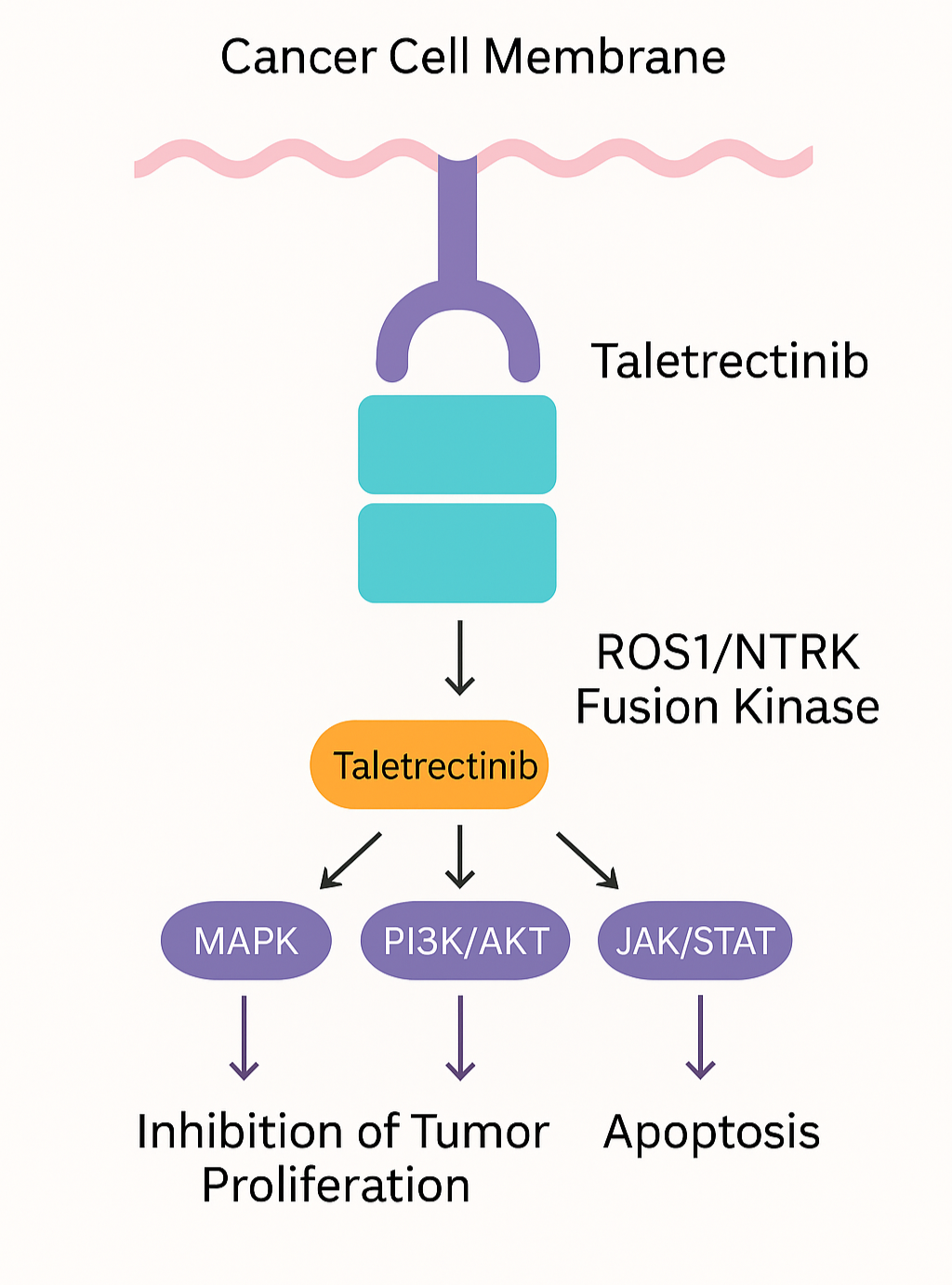
- Taletractinib works by selectively inhibiting the ROS1 and NTRK gene fusion kinases, which play a key role in tumor growth and survival. ROS1 and NTRK rearrangements lead to the production of abnormal proteins that drive cancer progression.
- Taletractinib blocks these abnormal kinases, inhibiting downstream signaling pathways such as MAPK, PI3K/AKT, and JAK/STAT, ultimately leading to apoptosis (programmed cell death) and inhibition of tumor spread.
- In addition, taletractinib has central nervous system (CNS) penetration, making it potentially effective in brain metastases, a common complication in ROS1-positive NSCLC.
Indications and Uses :
While still under clinical development, Taletrectinib is primarily being studied for the treatment of:
- ROS1-positive Non-Small Cell Lung Cancer (NSCLC) – including patients previously treated with crizotinib
- NTRK fusion-positive solid tumors – in various cancer types like colorectal, breast, or pancreatic cancer
As of now, Taletrectinib has not received full FDA approval, but it has shown promise in early-phase trials and is currently undergoing phase 2 and phase 3 clinical studies in the US, Japan, and China.
Taletrectinib Dosage and Administration :
Since Taletrectinib is still under investigation, the recommended dosage is based on clinical trials. In ongoing studies, the dosage ranges from 400 mg to 800 mg orally once daily, depending on the patient population and tolerance.
Administration Tips:
- It is taken orally with or without food
- Tablets should be swallowed whole, not crushed or chewed
- Always follow the investigator or trial sponsor’s guidelines if part of a clinical study
Patients should not alter the dosage on their own and must report any side effects to the trial coordinator or healthcare provider.
Clinical Trials and Efficacy Data :
Taletrectinib has shown encouraging results in phase 1 and 2 studies:
Key Highlights:
- In ROS1-positive NSCLC patients:
- Overall Response Rate (ORR): Up to 66%
- Duration of Response (DoR): Over 24 weeks
- Effective even in crizotinib-resistant patients
- Demonstrated blood-brain barrier penetration, reducing CNS lesions
The ongoing TRUST trial and TACTIC study are further evaluating its safety and efficacy across a broader population.
Side Effects of Taletrectinib :
Like all targeted therapies, Taletrectinib may cause adverse effects, though many are manageable. Reported side effects include:
| Common Side Effects | Frequency |
|---|---|
| Nausea | Very Common |
| Diarrhea | Common |
| Fatigue | Common |
| Elevated liver enzymes | Common |
| Dizziness | Occasionally |
| QT interval prolongation | Rare |
Patients on Taletrectinib should undergo routine liver function tests and ECG monitoring to check for early signs of hepatotoxicity and cardiac issues.
Precautions and Warnings :
Before starting Taletrectinib therapy, consider the following:
- Pregnancy & Breastfeeding: Not recommended due to potential fetal harm
- Liver impairment: Dose adjustments may be needed
- Drug Interactions: Avoid strong CYP3A4 inhibitors or inducers
- QT Prolongation: Monitor heart rhythm in patients with a history of cardiac issues
Always inform your healthcare provider of any other medications or supplements you’re taking.
Conclusion :
Taletractinib is emerging as a promising targeted therapy for patients with ROS1-positive NSCLC and NTRK fusion-positive cancers. Its dual-targeted inhibition, efficacy in resistant cases, and CNS penetration make it a promising alternative to older TKIs such as crizotinib.
While awaiting approval, ongoing trials suggest that taletractinib could be a game-changer in the precision oncology landscape. For patients and providers with difficult-to-treat lung cancer, taletractinib offers new hope.
Frequently Asked Questions (FAQs)
Q1. Is Taletrectinib FDA approved?
Q2. What cancers can Taletrectinib treat?
Q3. How is Taletrectinib different from Crizotinib?
Q4. What is the mechanism of action of Taletrectinib?
Q5. Can Taletrectinib be used in children?
Disclaimer: This blog post is for informational purposes only and does not constitute medical advice. Always consult a qualified healthcare professional before making any medical decisions.

1 thought on “Taletrectinib: A Powerful ROS1 Inhibitor for Lung Cancer – Complete Drug Guide”