Table of Contents
The year 2025 marked a historic moment for nephrology with the FDA approval of sibeprenlimab, bringing new hope to patients with primary IgA nephropathy (eGAN) – a chronic and progressive autoimmune kidney disorder that can lead to kidney failure. For decades, treatment options for IgA nephropathy have focused primarily on symptom management, blood pressure control, and delaying progression. However, there was a dire need for targeted therapies that address the underlying disease mechanisms.
With the FDA approval of sibeprenlimab, that gap has finally been filled.
This blog provides a detailed, comprehensive guide explaining what sibeprenlimab is, why the FDA approved it, how it works, its clinical data, dosing, benefits, risks, and its transformative impact on kidney care. Whether you are a patient, pharmacist, doctor, or medical blogger, this guide covers everything you need to know.
Introduction to IgA Nephropathy: Why Sibeprenlimab Matters
IgA nephropathy (IgAN), also known as Buerger’s disease, is a chronic immune-mediated kidney condition in which the body produces abnormal immunoglobulin A (IgA) that accumulates in the kidneys. Over time, these deposits damage the glomeruli and impair kidney function.
Common symptoms include:
• Persistent proteinuria
• Hematuria
• High blood pressure
• Fatigue
• Edema
• Gradual decline in renal filtration
IgAN is one of the leading causes of chronic kidney disease (CKD) worldwide. Until recently, treatment strategies included:
• ACE inhibitors or ARBs
• Steroids
• Immunosuppressive therapy
• Lifestyle changes
However, none of these target the underlying cause: abnormal IgA production.
That’s why the sibeprenlimab FDA approval is variable.
What Is Sibeprenlimab? A New Class of Kidney Therapeutics
Sibeprenlimab is a humanized monoclonal antibody developed specifically for IgA nephropathy. Unlike steroids or common immunosuppressants, sibeprenlimab works by selectively blocking APRIL (an inducer-inducing ligand) – a key cytokine involved in IgA production.
Its approval represents the first significant shift towards precision-targeted therapy for IgAN.
Key features of sibeprenlimab
• Targets the APRIL pathway
• Reduces circulating Gd-IgA1 levels
• Reduces proteinuria
• Slows decline in kidney function
• Robust clinical trial performance
• FDA-approved for adult patients with primary IgAN
The FDA approval of sibeprenlimab came under an accelerated approval pathway, reflecting its significant unmet need and strong clinical benefits.
Why the FDA Approved Sibeprenlimab: The Science Behind the Decision
The FDA’s decision to approve sibeprenlimab was driven by compelling clinical evidence demonstrating that the drug:
1. Significantly reduces proteinuria
Proteinuria is the strongest indicator of disease progression in IgAN. Sibeprenlimab therapy showed meaningful reductions in protein leakage into urine.
2. Slows down kidney damage
Trials showed improved eGFR (estimated glomerular filtration rate) slopes, reflecting slower kidney decline.
3. Targets the biological root cause
By inhibiting APRIL, sibeprenlimab decreases the production of abnormal IgA molecules—something no previous therapy could do directly.
4. Demonstrated safety and tolerability
The FDA noted the drug was well-tolerated with manageable side effects.
5. Meets urgent therapeutic need
Millions worldwide face IgAN with limited options. The sibeprenlimab FDA approval opens a completely new therapeutic pathway.
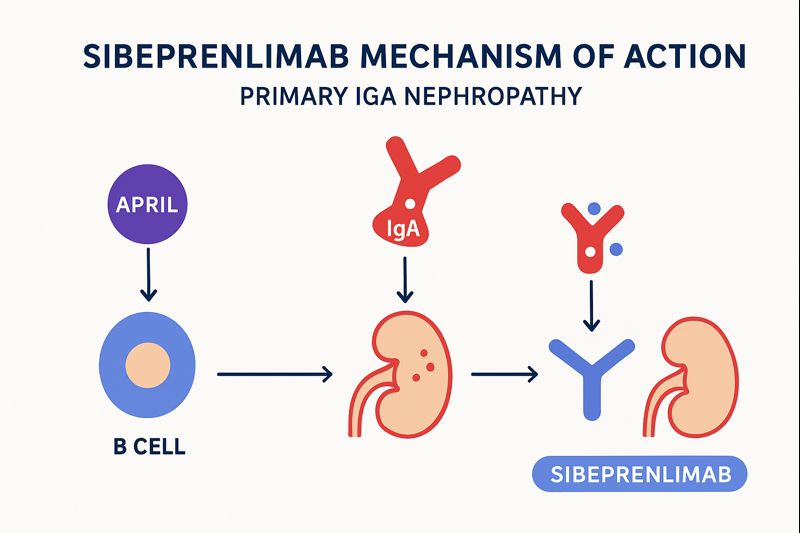
Sibeprenlimab Mechanism of Action (MOA): How the Drug Works
Sibeprenlimab works by blocking APRIL, a cytokine that is essential for the survival and activity of B cells that produce IgA antibodies.
Mechanism explained step by step
1. APRIL binds to B cells and plasma cells
→ Promotes the production of IgA antibodies
2. In IgAN patients, APRIL is overactive, leading to abnormal IgA production
3. These IgA molecules (specifically Gd-IgA1) accumulate in the kidneys
→ Causes inflammation and scarring
4. Sibeprenlimab binds to APRIL, inactivating it
5. This reduces abnormal IgA in the circulation
6. Decreased IgA accumulation
→ Reduced renal inflammation
→ Reduced proteinuria
→ Improved renal outcomes
Therefore, the FDA approval of sibeprenlimab reinforces how targeting APRIL offers a disease-modifying approach rather than symptomatic control.
Clinical Trial Data Leading to FDA Approval
The key trial supporting the sibeprenlimab FDA approval was a Phase 2 randomized, double-blind, placebo-controlled study assessing:
- Reduction in proteinuria
- Change in eGFR slope
- Biomarker response (Gd-IgA1 levels)
- Safety profile
Major Findings from Clinical Trials
🔥 40–50% reduction in proteinuria across high doses
🔥 Slowing of eGFR decline by up to 80%
🔥 Significant drop in pathogenic IgA biomarkers
🔥 Strong correlation between APRIL inhibition and renal protection
The FDA determined that these results were clinically meaningful, especially for a disease with limited treatment options.
Benefits & Advantages After Sibeprenlimab FDA Approval
The approval offers a new direction for IgAN care. Patients now gain access to:
1. A targeted mechanism—first in class for IgAN
Unlike steroids, sibeprenlimab is a precision therapy that directly targets disease biology.
2. Reduction in proteinuria
The strongest predictor of kidney failure is significantly improved.
3. Slower disease progression
Preserves kidney function over time.
4. Steroid-sparing effect
Reduces reliance on corticosteroids and immunosuppressants.
5. Better quality of life
Fewer relapses, improved kidney health, and reduced symptoms.
6. Safe and well-tolerated
Low incidence of serious infections or immunosuppression-related complications.
The sibeprenlimab FDA approval represents years of research leading to real-world patient benefits.
Dosage, Administration & Treatment Protocol
Sibeprenlimab is administered as:
- Subcutaneous injection
- Once monthly (depending on final FDA label)
- Nursing or clinic visits may be required depending on local guidelines
The monthly schedule helps improve adherence compared to oral medications or steroids requiring daily dosing.
Safety Profile and Side Effects
Clinical trials associated with the sibeprenlimab FDA approval reported a favorable safety profile.
Common Side Effects
- Injection site reactions
- Headache
- Fatigue
- Upper respiratory tract infections
- Mild gastrointestinal disturbances
Rare Side Effects
- Hypersensitivity reactions
- Reduced immunoglobulin levels
- Increased risk of viral infections
Regular monitoring of kidney function, IgA levels, and overall immune status is recommended.
Sibeprenlimab vs. Other IgAN Treatments
Before sibeprenlimab FDA approval, treatment options included:
- Steroids
- ACE inhibitors / ARBs
- SGLT2 inhibitors
- Immunosuppressants
- Fish oil
- Lifestyle modifications
How Sibeprenlimab Stands Out
| Feature | Before Sibeprenlimab | After Sibeprenlimab FDA Approval |
| Targeted therapy | ❌ No | ✅ Yes |
| Treats root cause | ❌ | ✅ APRIL pathway inhibition |
| Reduces abnormal IgA | ❌ | ✅ Significant |
| Durable proteinuria reduction | ⚠️ Variable | ⭐ Strong |
| Slows kidney decline | Moderate | Excellent compared to placebo |
This makes the sibeprenlimab FDA approval a major therapeutic advancement.
Cost, Accessibility & Insurance Coverage
Because sibeprenlimab is a biologic therapy, pricing may be significant. However:
- Insurance is expected to cover sibeprenlimab due to FDA approval
- Manufacturer patient assistance programs will be available
- Chronic kidney disease markets get strong reimbursement support
More details will become available as 2025 progresses.
Impact of Sibeprenlimab FDA Approval on Nephrology in 2025
The approval has already begun to influence:
1. Clinical guidelines
Nephrology societies are expected to integrate APRIL inhibitors in IgAN care pathways.
2. Research directions
New trials will compare sibeprenlimab with other biologics for combination therapy.
3. Patient outcomes
A measurable reduction in end-stage renal disease (ESRD) risk is anticipated.
The sibeprenlimab FDA approval is not just a new drug—it represents a shift toward biologics in kidney diseases.
Is Sibeprenlimab Right for You?
Best suited for:
- Adults with primary IgA nephropathy
- Patients with persistent proteinuria
- Individuals not achieving results with ACE inhibitors or SGLT2 inhibitors
- Those seeking targeted biologic therapy
Not recommended for:
- People with severe immunodeficiency
- Patients with active severe infection
- Pregnant or breastfeeding women (risk not yet established)
Always consult a nephrologist before starting therapy.
Future Prospects After Sibeprenlimab FDA Approval
The approval opens the pathway for:
- Global rollouts beyond the U.S.
- Combination biologic therapy trials
- Improved patient monitoring technologies
- Real-world evidence studies
It is likely to inspire new APRIL-targeting drugs.
Conclusion
The FDA approval of sibeprenlimab in 2025 is one of the most significant advances in the treatment of IgA nephropathy. For the first time, this therapy directly targets the biological driver of the disease – the excessive abnormal IgA production caused by APR.
With its potential to reduce proteinuria, slow kidney decline, and improve long-term outcomes, sibeprenlimab brings new hope to millions of people struggling with this chronic disease.
As more data emerges from ongoing trials and real-world use, sibeprenlimab is expected to reshape nephrology treatment standards worldwide.