Table of Contents
Introduction
Targeted cancer therapy has transformed modern oncology by shifting treatment strategies from non-specific cytotoxic chemotherapy to precision-driven molecular inhibition. One of the most significant advancements in this space is the development of PARP inhibitors, including rucaparib. Understanding the rucaparib mechanism of action is essential for healthcare professionals, pharmacists, oncology researchers, and students who want a clear understanding of how this targeted therapy works at a cellular level.
Rucaparib is an orally administered small-molecule inhibitor primarily used in ovarian, fallopian tube, and primary peritoneal cancers associated with BRCA mutations or homologous recombination deficiency (HRD). The rucaparib mechanism of action revolves around the selective inhibition of PARP enzymes, which play a crucial role in DNA repair pathways.
This in-depth guide explores the molecular basis, clinical relevance, safety profile, regulatory history, and pharmacological considerations of the rucaparib mechanism of action, presented in a structured and clinically focused format.
Overview of Rucaparib
Rucaparib belongs to the class of drugs known as PARP inhibitors (Poly ADP-Ribose Polymerase inhibitors). These drugs exploit synthetic lethality in tumor cells that already possess defects in DNA repair mechanisms, particularly BRCA1 and BRCA2 mutations.
Approved for specific ovarian cancer indications, rucaparib is administered orally and offers a targeted treatment approach that interferes with tumor cell survival while sparing many normal cells.
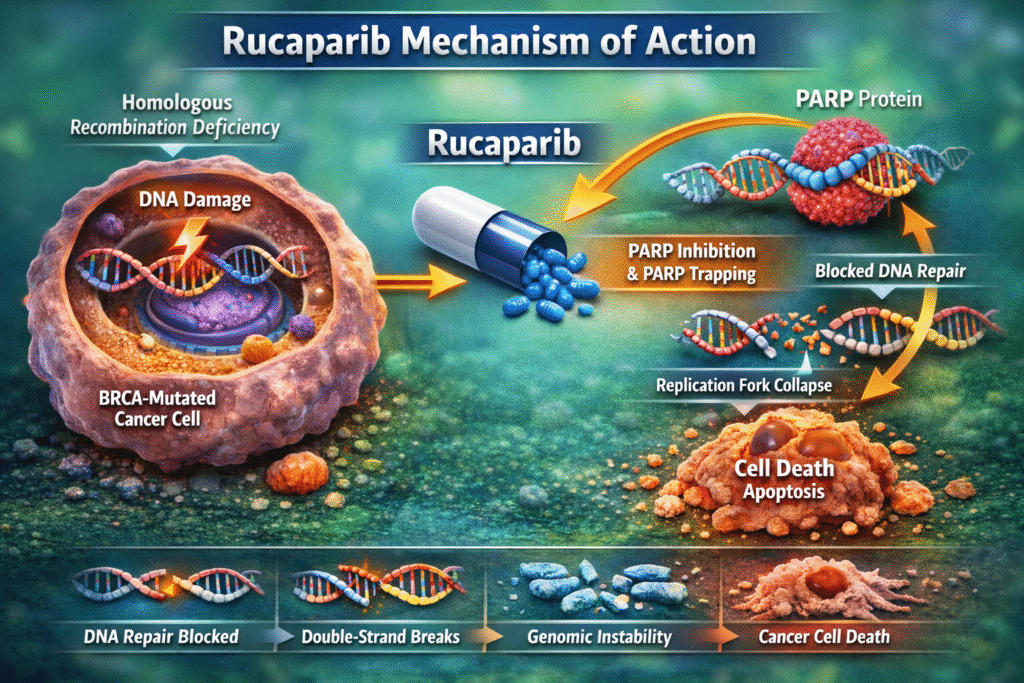
The therapeutic foundation of rucaparib is directly linked to the rucaparib mechanism of action, which disrupts DNA damage repair processes in cancer cells.
Molecular Basis of Rucaparib Mechanism of Action
Understanding the rucaparib mechanism of action requires first understanding DNA repair biology.
Role of PARP Enzymes in DNA Repair
PARP-1, PARP-2, and PARP-3 are enzymes responsible for repairing single-strand DNA breaks through the base excision repair (BER) pathway. When DNA single-strand breaks occur:
- PARP detects DNA damage.
- PARP binds to damaged DNA.
- PARP catalyzes poly ADP-ribosylation.
- DNA repair proteins are recruited.
- DNA repair is completed.
This repair system maintains genomic stability.
What Happens When PARP Is Inhibited?
The rucaparib mechanism of action involves:
- Competitive inhibition of PARP catalytic activity.
- Prevention of PARP-mediated DNA repair.
- Trapping PARP enzymes on DNA strands.
- Conversion of single-strand breaks into double-strand breaks during replication.
In cancer cells with BRCA mutations, homologous recombination repair is already defective. Therefore, when rucaparib inhibits PARP, the cancer cell cannot repair DNA damage effectively, leading to genomic instability and apoptosis.
This dual effect—enzymatic inhibition and PARP trapping—is central to the rucaparib mechanism of action.
Synthetic Lethality and Targeted Cancer Therapy
One of the most important concepts behind the rucaparib mechanism of action is synthetic lethality.
Synthetic lethality occurs when:
- Pathway A is defective (e.g., BRCA mutation).
- Pathway B is inhibited (e.g., PARP inhibition by rucaparib).
- The combination results in cell death.
Normal cells with functional BRCA genes can survive PARP inhibition because they retain homologous recombination repair capability. Cancer cells with BRCA mutations cannot, which makes the rucaparib mechanism of action selectively toxic to tumor cells.
Cellular Effects of Rucaparib
The rucaparib mechanism of action produces several downstream effects:
- Accumulation of DNA damage
- Replication fork collapse
- Chromosomal instability
- Cell cycle arrest
- Apoptotic cell death
Additionally, rucaparib enhances cytotoxicity in tumors with:
- BRCA1 mutations
- BRCA2 mutations
- Homologous recombination deficiency
- Genomic instability signatures
These biological effects validate the clinical significance of the rucaparib mechanism of action.
Clinical Applications Linked to Rucaparib Mechanism of Action
The rucaparib mechanism of action makes it particularly effective in:
- Recurrent ovarian cancer
- BRCA-mutated ovarian cancer
- Platinum-sensitive ovarian cancer
- Maintenance therapy following response to chemotherapy
Patients are typically tested for BRCA mutations or HRD status before therapy initiation.
Pharmacodynamics of Rucaparib
The pharmacodynamic properties are closely related to the rucaparib mechanism of action:
- Dose-dependent PARP inhibition
- Sustained PARP trapping activity
- Tumor-selective cytotoxicity
- Reduced DNA repair capacity
PARP trapping potency differentiates rucaparib from some other agents in its class.
FDA Approval and Regulatory History
On December 17, 2025, the Food and Drug Administration (FDA) approved rucaparib for specific ovarian cancer indications based on clinical trial data demonstrating objective response rates and progression-free survival improvement.
The approval was based on:
- Demonstrated efficacy in BRCA-mutated ovarian cancer
- Durable response rates
- Manageable safety profile
The regulatory decision reflects the strength of the rucaparib mechanism of action in targeting molecularly defined tumors.
Side Effects of Rucaparib
Like all anticancer therapies, rucaparib has associated adverse effects.
Common side effects include:
- Nausea
- Fatigue
- Anemia
- Elevated liver enzymes
- Vomiting
- Decreased appetite
- Thrombocytopenia
Serious but less common adverse effects:
- Myelodysplastic syndrome (MDS)
- Acute myeloid leukemia (AML)
- Severe hematologic toxicity
The side effect profile relates indirectly to the rucaparib mechanism of action, as bone marrow cells also rely on DNA repair processes.
Monitoring Parameters
Due to the biological activity linked to the rucaparib mechanism of action, patients require:
- Complete blood count monitoring
- Liver function tests
- Renal function evaluation
- Assessment for signs of bone marrow suppression
Early detection of toxicity ensures safer treatment continuation.
Drug Interactions
Rucaparib may interact with:
- CYP enzyme substrates
- Strong CYP inhibitors
- Strong CYP inducers
- Drugs affecting hepatic metabolism
Because the rucaparib mechanism of action depends on adequate systemic drug exposure, altered metabolism can influence therapeutic outcomes.
Clinicians should evaluate:
- Concomitant medications
- Herbal supplements
- Anticoagulants
- Hormonal therapies
Careful medication review reduces interaction risks.
Comparison With Other PARP Inhibitors
Although multiple PARP inhibitors exist, differences include:
- PARP trapping potency
- Dosing schedules
- Indication approvals
- Toxicity profiles
However, the foundational principle of the rucaparib mechanism of action remains consistent: inhibition of DNA repair via PARP blockade.
Resistance Mechanisms
Cancer cells may develop resistance through:
- BRCA reversion mutations
- Restoration of homologous recombination
- Increased drug efflux
- PARP mutation
Understanding resistance patterns is crucial to overcoming limitations of the rucaparib mechanism of action in long-term therapy.
Pharmacokinetics Overview
Key characteristics:
- Oral bioavailability
- Hepatic metabolism
- Moderate half-life
- Steady-state concentrations achieved with repeated dosing
Pharmacokinetic stability ensures sustained engagement of the rucaparib mechanism of action.
Clinical Trial Insights
Clinical trials demonstrated:
- Improved progression-free survival
- Higher response rates in BRCA-mutated patients
- Benefit as maintenance therapy
- Manageable toxicity
These findings reinforce the therapeutic relevance of the rucaparib mechanism of action in precision oncology.
Future Research Directions
Ongoing investigations explore:
- Combination therapies with immunotherapy
- Use in other HRD-positive tumors
- Biomarker refinement
- Overcoming drug resistance
The scientific community continues to explore expanded applications of the rucaparib mechanism of action.
Practical Considerations for Healthcare Professionals
Before initiating therapy:
- Confirm genetic testing
- Assess baseline labs
- Evaluate comorbidities
- Counsel patients about side effects
Understanding the rucaparib mechanism of action allows clinicians to personalize treatment strategies effectively.
Conclusion
The rucaparib mechanism of action represents a milestone in targeted oncology therapy. By inhibiting PARP enzymes and exploiting synthetic lethality in BRCA-mutated and HRD-positive tumors, rucaparib provides a precise and biologically rational treatment strategy.
Its clinical success underscores the power of molecularly guided therapy in improving cancer outcomes. While associated with manageable side effects and specific drug interactions, proper patient selection and monitoring optimize safety and efficacy.
As oncology continues evolving toward personalized medicine, the rucaparib mechanism of action remains a cornerstone example of how understanding cellular biology can directly translate into life-extending therapies.