Table of Contents
Introduction
Vision is one of the most vital human senses, yet millions of people worldwide face progressive vision loss due to retinal diseases. Conditions such as age-related macular degeneration, diabetic macular edema, and retinal vein occlusion are major contributors to irreversible blindness if left untreated. Over the last two decades, the development of anti-VEGF therapies has revolutionized ophthalmology. Among these therapies, ranibizumab-leyk has emerged as an important and reliable treatment option.
Ranibizumab-leyk is a modern biologic therapy designed to target the underlying molecular mechanisms responsible for abnormal blood vessel growth in the retina. Rather than simply managing symptoms, this therapy intervenes at the disease pathway level, helping to slow progression, preserve vision, and improve quality of life.
With FDA approval and increasing clinical adoption, ranibizumab-leyk is gaining recognition as a safe, effective, and accessible option in retinal disease management.
What Is Ranibizumab-leyk?
Ranibizumab-leyk (brand name Nufymco) is a recombinant humanized monoclonal antibody fragment (Fab) specifically engineered to inhibit vascular endothelial growth factor A (VEGF-A). VEGF-A is a key signaling protein that promotes abnormal angiogenesis and vascular leakage within the retina.
In healthy eyes, VEGF plays a controlled role in maintaining blood vessel function. However, in retinal diseases, VEGF becomes overexpressed, leading to fragile, leaky blood vessels that damage retinal tissue. Ranibizumab-leyk neutralizes this excess VEGF, preventing further retinal injury.
From a regulatory standpoint, Nufymco is developed to meet stringent standards of quality, safety, and efficacy, ensuring consistency with established anti-VEGF therapies.
Why VEGF Inhibition Matters in Retinal Diseases
Understanding the importance of ranibizumab-leyk requires an appreciation of VEGF’s role in ocular pathology.
When retinal tissue experiences hypoxia or inflammation:
- VEGF levels increase dramatically
- New blood vessels form abnormally
- These vessels are weak and prone to leakage
- Fluid accumulates in the macula
- Vision becomes distorted or blurred
By blocking VEGF-A activity, ranibizumab-leyk directly interrupts this disease cascade.
Clinical Uses
Nufymco is prescribed for multiple retinal conditions where VEGF-driven pathology plays a central role.
1. Wet Age-Related Macular Degeneration (AMD)
In wet AMD, abnormal blood vessels grow beneath the retina and macula, leading to rapid vision loss. Ranibizumab-leyk suppresses this abnormal angiogenesis, helping stabilize or improve visual acuity.
2. Diabetic Macular Edema (DME)
Chronic hyperglycemia damages retinal blood vessels in diabetic patients. Ranibizumab-leyk reduces macular swelling by limiting VEGF-mediated leakage.
3. Diabetic Retinopathy
By slowing disease progression, Nufymco helps preserve retinal structure in patients with advanced diabetic eye disease.
4. Retinal Vein Occlusion (RVO)
Blocked retinal veins cause fluid accumulation and ischemia. Nufymco reduces edema and improves visual outcomes.
5. Myopic Choroidal Neovascularization (mCNV)
In pathological myopia, ranibizumab-leyk helps control abnormal vessel growth that threatens central vision.
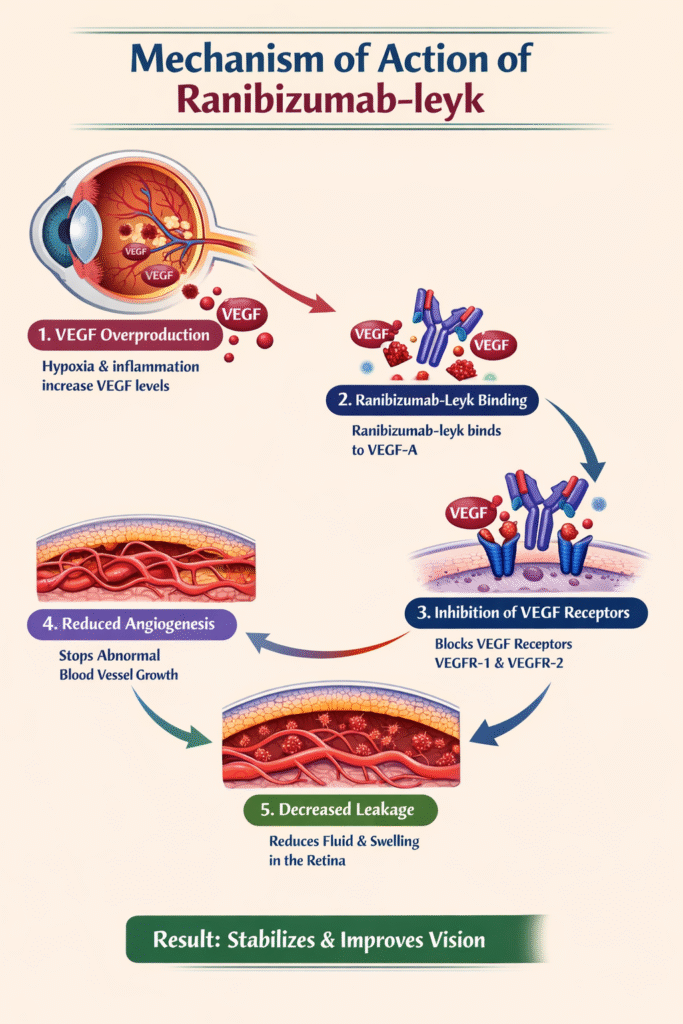
Mechanism of Action
The mechanism of action of Nufymco is highly specific and biologically targeted.
Step-by-Step Explanation
- VEGF Overproduction
Retinal hypoxia and inflammation trigger excess VEGF-A production. - VEGF Binding
Ranibizumab-leyk binds directly to VEGF-A with high affinity. - Receptor Blockade
VEGF-A can no longer activate VEGF receptors (VEGFR-1 and VEGFR-2). - Angiogenesis Suppression
New abnormal blood vessel formation is halted. - Reduced Vascular Leakage
Fluid leakage into the macula decreases.
This targeted action explains why ranibizumab-leyk is effective even in chronic and progressive retinal disorders.
Pharmacokinetics and Localized Action
After intravitreal injection, ranibizumab-leyk remains localized within the eye. Systemic absorption is minimal, which significantly reduces the risk of systemic adverse effects. This localized pharmacokinetic profile makes Nufymco suitable for long-term use.
Dosage and Administration
Nufymco is administered exclusively via intravitreal injection by trained ophthalmologists.
Typical Dosing Regimen
- Dose: 0.5 mg per injection
- Route: Intravitreal
- Initial phase: Monthly injections
- Maintenance: PRN or treat-and-extend approach
Strict aseptic technique is essential to minimize injection-related complications when using ranibizumab-leyk.
Side Effects
Like all intravitreal therapies, ranibizumab-leyk is associated with certain side effects. Most are mild and manageable.
Common Side Effects
- Eye pain or irritation
- Conjunctival hemorrhage
- Transient increase in intraocular pressure
- Visual floaters
- Eye redness
Less Common but Serious Adverse Effects
- Endophthalmitis
- Retinal detachment
- Severe intraocular inflammation
- Cataract formation
- Thromboembolic events (rare)
Regular follow-up ensures early detection and management of potential complications related to ranibizumab-leyk.
Safety Profile and Monitoring
The safety profile of Nufymco is well-established. Patients typically undergo:
- Visual acuity testing
- Optical coherence tomography (OCT)
- Intraocular pressure measurement
These evaluations help assess treatment response and maintain patient safety.
FDA Approval of Nufymco
The FDA approval of Nufymco was granted after comprehensive evaluation of:
- Analytical similarity
- Clinical efficacy
- Safety outcomes
- Immunogenicity risk
The FDA concluded that Nufymco demonstrates no clinically meaningful differences compared to reference therapies. This approval ensures confidence among clinicians and patients alike.
Drug Interaction Profile
Because ranibizumab-leyk is administered locally into the eye, systemic drug interactions are minimal.
Important Considerations
- Caution when combining with other intravitreal agents
- Avoid simultaneous bilateral injections unless necessary
- Monitor patients on systemic anti-VEGF therapy
To date, no significant systemic drug interactions have been documented with ranibizumab-leyk.
Comparison with Other Anti-VEGF Agents
| Feature | Ranibizumab-leyk | Bevacizumab | Aflibercept |
|---|---|---|---|
| Target | VEGF-A | VEGF-A | VEGF-A, PlGF |
| Administration | Intravitreal | Intravitreal (off-label) | Intravitreal |
| FDA Approved | Yes | No (ocular) | Yes |
| Biosimilar | Yes | No | No |
Cost, Accessibility, and Patient Benefits
As a follow-on biologic, ranibizumab-leyk improves access to advanced retinal therapy. Reduced treatment costs can enhance patient adherence, leading to better long-term visual outcomes.
Future Outlook of Nufymco
The future role of Nufymco appears promising due to:
- Rising prevalence of diabetic eye disease
- Aging global population
- Increasing real-world evidence
- Broader healthcare adoption
As innovation continues, Nufymco is expected to remain a cornerstone therapy in retinal disease management.
Conclusion
Ranibizumab-leyk represents a major advancement in the treatment of retinal diseases driven by VEGF overexpression. Its targeted mechanism, strong safety profile, FDA approval, and improved accessibility make ranibizumab-leyk a valuable option for preserving vision and enhancing patient outcomes.