Table of Contents
Introduction :
The Pharmacovigilance Master File also called as Pharmacovigilance System Master File (PSMF) is a legally required document that outlines a pharmaceutical company’s entire drug testing system. It is a key component in complying with global regulations and ensuring patient safety.
In this post, you will find the structure, sections, processes and best practices of a PSMF, as well as tips for being prepared for inspections in 2025 and beyond.
What Is the Pharmacovigilance Master File?
The PSMF provides a detailed description of a company’s pharmacovigilance (PV) system and documents how drug safety activities are organized, monitored, and controlled.
It is mandatory in the EU and many other countries, including the UK, Saudi Arabia, and India, and is used for:
• Regulatory inspections
• QPPV monitoring
• System audits
• Demonstrating transparency in safety processes
Legal Requirement for the PSMF :
Under EU Regulation (EC) No 726/2004 and Directive 2001/83/EC, all Marketing Authorisation Holders (MAHs) are required to:
• Keep an up-to-date PSMF
• Store it at the location of the QPPV
• Make it available to authorities upon request
• Ensure that its content accurately reflects the current PV system
Other countries have adopted or mirrored this requirement to enhance global drug monitoring consistency.
Key Sections of the Pharmacovigilance Master File :
The PSMF has two major parts:
Main Body (Core Information) –
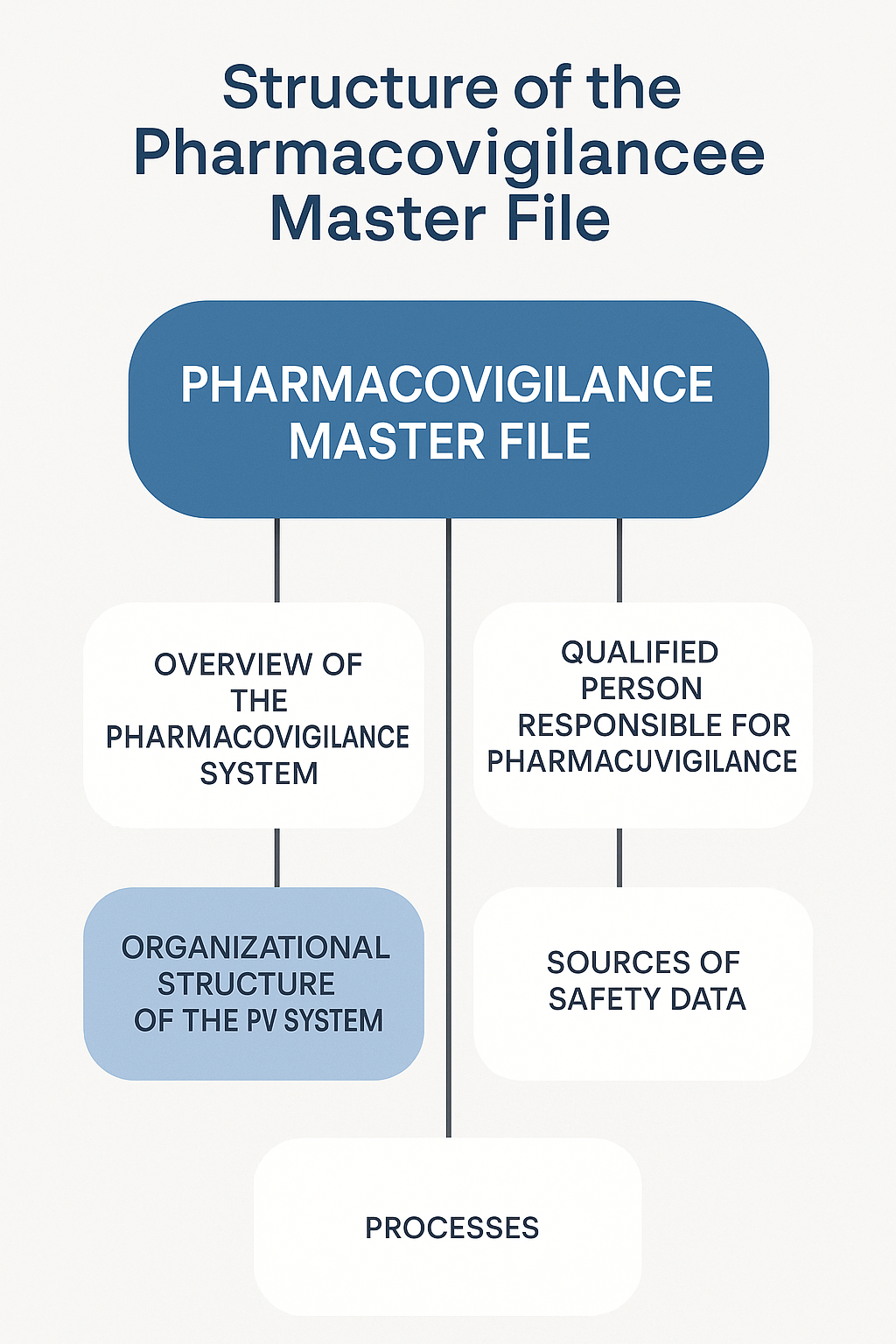
Section 1: Overview of Pharmacovigilance System
Describes the general framework, objectives and purpose of the company’s pharmacovigilance practices.
Section 2: Qualified Person for Pharmacovigilance (QPPV)
Includes contact details, qualifications and legal responsibilities of the QPPV, including backup arrangements.
Section 3: Organizational Structure
Provides an organization chart and outlines how pharmacovigilance activities are distributed across departments and external vendors.
Section 4: Sources of Safety Data
Includes all data collection sources such as:
• Spontaneous ADRs
• Clinical trial reports
• Literature surveillance
• Market research
• Digital/social media monitoring
Section 5: Computerized Systems
Lists all databases and tools used to record, manage and analyze drug testing data.
Section 6: Quality System
Describes:
• SOPs (Standard Operating Procedures)
• Employee Training and Qualification
• Deviation Handling
• Corrective and Preventive Actions (CAPAs)
• Internal Audit Program
Section 7: Performance Metrics
Shows how performance is tracked through KPIs such as:
• Case Processing Times
• Adherence to Reporting Timelines
• Signal Detection Frequency
Section 8: Contractual Agreements
Outlines Security Data Exchange Agreements (SDEAs) and partnerships with CROs, affiliates, or third-party vendors.
Annexes (Supporting Documentation) –
Annex A: Product List
Details of all products covered under the PSMF
Annex B: SOPs
List of all PV-related procedures
Annex C: Organizational Charts
Visual charts of teams and reporting lines
Annex D: Training Records
Certification and training logs for staff
Annex E: Audit Reports
Internal and external audits, with CAPAs
Annex F: Agreements
Contracts with external PV service providers
Annex G: KPIs and Metrics
Performance data of the PV system
Structure and Process of the Pharmacovigilance Master File :
Understanding how the PSMF is structured and maintained helps organizations stay compliant and efficient. Here’s how it works:
A. Structural Framework –
| Component | Description |
| Main Body | Core narrative of the PV system’s framework and responsibilities |
| Annexes | Live documents providing operational details, data, and evidence |
| Index Table | Optional but useful; acts as a table of contents for navigation |
| Change Log | Records version history and updates made to each section |
B. PSMF Process Flow –
- Data Collection
– Safety data from all sources is gathered in real time. - Internal Communication
– Updates are shared with the QPPV and responsible departments. - Document Update
– Main body is reviewed annually; annexes are updated quarterly or as needed. - Version Control
– Each change is documented with timestamps and author identification. - Audit and Review
– Internal audits verify accuracy and alignment with PV activities. - Regulatory Access
– The PSMF is submitted or presented during inspections and upon request.
⚙️ Tools to Support the Process
- Document Management Systems (DMS)
- Workflow automation platforms
- PV database integration (e.g., Argus, ARISg)
Update Frequency and Version Control :
| Component | Update Frequency |
| Main Body | Annually or upon major system change |
| Annexes | Quarterly or as needed |
| Change Log | With each version update |
Make sure updates are signed by the QPPV and tracked with proper document control.
Regulatory Inspections & Readiness :
During inspections, health authorities examine whether:
- The PSMF reflects actual system operations
- The QPPV has oversight and authority
- Quality systems are functional and followed
- Performance data is accurately tracked and reported
Tip: Keep a PSMF summary and electronic access folder ready for quick sharing.
Best Practices :
• Maintain a live PSMF dashboard
• Conduct biannual PSMF audits
• Align PSMF with your PV training matrix
• Automate version control and update notifications • Use infographics and flowcharts for quick reference during inspections
Conclusion :
The Pharmacovigilance Master File is a fundamental document that ensures your company’s commitment to patient safety, regulatory transparency, and ongoing compliance. By clearly designing the PSMF, maintaining it regularly, and aligning it with real-time PV operations, your organization can be audit-ready and globally compliant in 2025 and beyond.
Frequently Asked Questions (FAQs) :
1. Is the Pharmacovigilance Master File (PSMF) mandatory outside the EU?
2. What is the difference between PSMF and RMP?
3. Who is responsible for the Pharmacovigilance Master File (PSMF)?
4. Can the PSMF be fully electronic?
5. What happens if my PSMF is outdated during an inspection?
Disclaimer:
This blog post is intended for informational and educational purposes only. It does not constitute legal, regulatory, or professional advice. Readers are encouraged to consult relevant regulatory authorities or qualified professionals before making decisions related to pharmacovigilance compliance or the preparation of a Pharmacovigilance Master File (PSMF). The content is based on publicly available sources and is not affiliated with any government or regulatory agency.

