Name of Drug : Penpulimab-kcqx
Drug Category : Antineoplastic agent
Form : Injection .
Table of Contents
Introduction :
Nasopharyngeal carcinoma (NPC) is a rare cancer that arises in the nasopharynx, most often seen in the non-keratinizing form linked to the Epstein Barr virus. In April 2025, the FDA approved a promising immunotherapy: penpulimab KCQX, a next-generation PD1 inhibitor, for the treatment of recurrent or metastatic non-keratinizing NPC – in combination with first-line combo chemotherapy and as a single agent in later lines.
What Is Penpulimab‑kcqx?
Penpulimab KCQX (also known as AK105 or simply “penpulimab”) is a PD1 inhibitor – an immune checkpoint blocker used to treat certain cancers. It is a humanized monoclonal antibody that is produced on an IgG1 Fc-null backbone, meaning that it avoids unwanted immune side effects and effectively enhances T cell activity by blocking the PD 1/PD L1/PD L2 pathway.
Originally approved in China (2021) for Hodgkin lymphoma and lung cancer, the FDA approved it in the United States on April 23, 2025, specifically for non-keratinizing nasopharyngeal carcinoma (NPC) – both as a first-line combination treatment and as a single agent in advanced settings.
Understanding Nasopharyngeal Carcinoma (NPC) :
Nasopharyngeal carcinoma arises from epithelial cells lining the back of the nose and upper part of the throat. Most NPC cases are non-keratinizing, are associated with Epstein-Barr virus (EBV) infection, and are generally more responsive to radiotherapy and chemotherapy. In advanced stages—recurrent or metastatic—treatment options are limited, and outcomes have historically been poor. Approximately 60,000 new head and neck cancers are diagnosed in the United States each year, with NPC accounting for a large proportion of the burden.
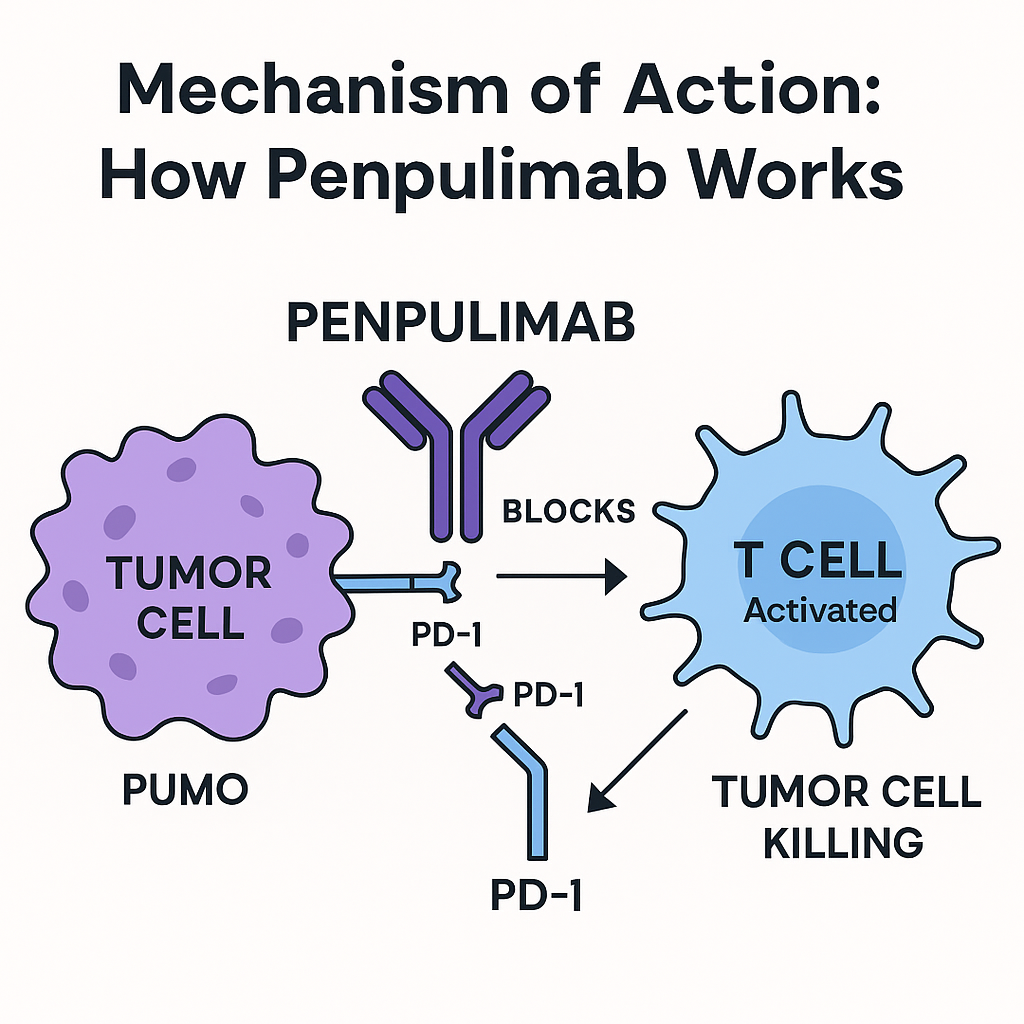
Mechanism of Action: How Penpulimab-kcqx Works ?
Penpulimab blocks the PD 1 receptor on T cells, preventing its interaction with PD-L1/PD-L2, and reactivating the anti-tumor immune response. Its specific Fc-null IgG1 structure reduces antibody-dependent cytotoxicity, which is intended to reduce some immune adverse effects and increase receptor coverage for robust tumor control.
FDA Approval Summary – April 23, 2025 :
The FDA cleared penpulimab‑kcqx for two scenarios:
1.First-line therapy (combo)
- Penpulimab‑kcqx + cisplatin or carboplatin + gemcitabine
- For adults with recurrent or metastatic non‑keratinizing NPC
2. Second-line or later (monotherapy)
- Single-agent penpulimab‑kcqx
For adults with metastatic non‑keratinizing NPC after progression on platinum-based chemo and at least one other prior therapy (The FDA also granted Fast Track, Breakthrough, and Orphan Drug designations, accelerating development due to clear unmet needs
Clinical Trials Behind the Approval :
Phase III: AK105‑304 (Combo Therapy)
- Design: Randomized, double-blind, global study (291 patients)
- Treatment: Penpulimab 200 mg IV q3 weeks + gemcitabine + cisplatin/carboplatin vs. placebo-containing regimen
- Results:
- Median progression-free survival (PFS): 9.6 months with penpulimab vs. 7.0 months with placebo (HR 0.45; 55% risk reduction; p < .0001) (Twelve-month PFS: 31% (penpulimab) vs. 11% (placebo) .At a median 19.1-month follow-up: 24-month PFS reached 22.8% vs. 5.5% in control .
- Overall survival (OS) data were immature but showed no negative trend.
Phase II: AK105‑202 (Monotherapy)
- Design: Single-arm, open-label in 125 patients
- Results:
- Overall response rate (ORR): 28% (95% CI: 20–37%)
- Duration of response (DOR): Not reached; at least 9.2 months
- 12-month DOR seen in about 46% of responders.
Dosage & Administration for Penpulimab-kcqx :
Based on FDA-approved prescribing info:
Combo therapy:
- Penpulimab-kcqx 200 mg IV over 60 min every 3 weeks, alongside gemcitabine (1,000 mg/m²) and either cisplatin (80 mg/m²) or carboplatin (AUC 5) for six cycles, followed by penpulimab alone up to 24 months.
Monotherapy (post-chemo):
- Penpulimab 200 mg IV over 60 min every 2 weeks, continued until progression or unacceptable toxicity, up to two years.
No dose reductions permitted; for immune-related AEs, treatment may be held or discontinued per guidelines .
Safety Profile: What You Should Know ?
Common combo AEs (≥20% frequency): nausea, vomiting, constipation, decreased appetite/weight, cough, fatigue, rash, fever, and notably hypothyroidism .
Common monotherapy AEs (≥20%): hypothyroidism, musculoskeletal pain; anemia also noted
Immune-mediated (rare but serious): pneumonitis, colitis, hepatitis, nephritis, endocrinopathies, skin reactions—treatment guidelines recommend steroids or discontinuation as needed .
Fatal AEs (≈1%): single cases of pneumonitis, septic shock, colitis, hepatitis .
Why It Matters ? Clinical & Real‑World Significance :
✅ A new front-line option
With its approval, penpulimab becomes the second FDA-approved PD‑1-based front-line therapy, joining toripalimab. The global AK105‑304 trial included patients from Asia, Latin America, Canada, and the U.S., enhancing confidence in its efficacy across diverse populations .
🎯 More patients can benefit
Prior to this, penpulimab was largely used in later lines or within China. Now, U.S. patients with recurrent or metastatic non‑keratinizing NPC have access to a powerful immunotherapy earlier in their treatment course .
🕒 Durable responses
A 55% reduction in progression or death compared with chemo alone is a significant clinical advance in a disease setting with poor historical outcomes .
Side Effects of Penpulimab-kcqx :
Common Side Effects of Penpulimab-kcqx –
These side effects were reported in ≥20% of patients:
When used in combination with chemotherapy (AK105-304 trial):
- Nausea
- Vomiting
- Fatigue
- Constipation
- Decreased appetite
- Weight loss
- Fever (pyrexia)
- Cough
- Rash
- Hypothyroidism
- Anemia
- Thrombocytopenia
Most were mild to moderate (Grade 1–2) in severity.
⚠️ Immune-Mediated Adverse Reactions –
Penpulimab-kcqx is an immune checkpoint inhibitor (PD-1 blocker), so it may cause the immune system to attack normal organs. These side effects can be serious or life-threatening if not treated early.
Reported immune-related effects include:
| Organ/System | Potential Side Effects |
|---|---|
| Lungs | Pneumonitis (inflammation) |
| Colon | Colitis (diarrhea, cramps) |
| Liver | Hepatitis (↑ALT/AST, jaundice) |
| Kidneys | Nephritis (↑creatinine) |
| Endocrine | Hypothyroidism, hyperthyroidism, adrenal insufficiency, type 1 diabetes |
| Skin | Severe rash, Stevens-Johnson syndrome |
| Nervous System | Encephalitis, Guillain-Barré (rare) |
These typically appear within weeks to months of starting therapy and require prompt corticosteroid treatment.
⚰️ Serious and Fatal Adverse Events –
In clinical trials, 1% of patients experienced treatment-related fatal adverse events, including:
- Pneumonitis
- Septic shock
- Colitis
- Hepatitis
⚠️ These cases were rare but highlight the need for close monitoring.
Considerations for Patients & Oncologists :
📋 Patient selection –
- First-line combo: Adults with recurrent/metastatic non-keratinizing NPC, no prior systemic therapy.
- Second-line monotherapy: Adults who progressed after platinum chemo and other prior lines.
🔍 Monitoring –
- Baseline tests: complete blood counts, liver/renal panels, thyroid function.
- Monitor for fatigue, GI symptoms, respiratory symptoms, endocrine issues.
- Treat immune-related AEs early with high-dose steroids; discontinue for severe cases
🔄 Duration of therapy –
- Combo: Up to 2 years.
- Monotherapy: Continue up to 24 months unless toxicity or progression occurs.
Future Outlook :
Given its first-line approval and favorable safety profile, Penpulimab-kcqx is poised to reshape treatment algorithms for recurrent/metastatic NPC in the coming years. Ongoing research explores its use across other tumor types, including lung cancer, lymphoma, and additional head and neck cancers .
Conclusion :
Penpulimab‑kcqx is a groundbreaking immunotherapy ally in both the first-line and later-line treatment of recurrent/metastatic non‑keratinizing NPC. Clinical trials like AK105‑304 and AK105‑202 show clear improvements in progression-free survival, response rates, and durable control, leading to FDA approvals in April 2025. With a manageable safety profile and a unique molecular design, Penpulimab-kcqx offers new hope to patients facing a challenging cancer with limited prior options.
FAQs :
1. What exactly is “non‑keratinizing” NPC?
2. How does Penpulimab-kcqx compare to other PD‑1 inhibitors?
3. Are there drug interactions?
4. What support programs are available?
5. Any new approved drug for Lung cancer in 2025 ?
Disclaimer:
This article is for informational and educational purposes only and does not substitute professional medical advice, diagnosis, or treatment. Always consult your healthcare provider before starting or changing any cancer treatment.

