Table of Contents
Introduction :
In the competitive pharmaceutical industry, patents and exclusivity are important tools used to protect new drug innovations and prevent competition in the marketplace. If you’re wondering why generic drugs are slow to arrive, how long branded drugs last on the market, or where to find U.S. regulations – understanding patent for pharmaceuticals is a good starting point.
This comprehensive guide explains the types of drug patents, the FDA exclusivity system, how long they last, and how they differ. You’ll also learn where to find these regulations in the Code of Federal Regulations (C.F.R.).
What is a patent for pharmaceuticals?
A patent for pharmaceuticals is legal protection granted by the United States Patent and Trademark Office (USPTO). It gives the holder the exclusive right to make, use, sell, or import a drug for a specified period of time—usually 20 years from the date of filing.
Patents are essential in the drug development lifecycle because they protect:
• New chemical compounds (active pharmaceutical ingredients or APIs)
• Manufacturing processes
• Drug formulations (e.g., extended-release tablets)
• Methods of use (e.g., treating a new disease with an old drug)
Types of Patent for Pharmaceuticals :
Patent for pharmaceuticals fall into several categories. Innovators often use multiple types of patents to cover various aspects of a drug product. These include:
1. Compound (Chemical Entity) Patents –
Protect the new drug molecule itself—often the most valuable and earliest-filed patent.
2. Formulation Patents –
Covers the way the drug is formulated—such as capsules, gels, or extended-release tablets.
3. Process Patents –
Protects the manufacturing method or synthesis pathway used to produce the drug.
4. Polymorph Patents –
Covers crystalline forms of a compound that may have different stability or solubility.
5. Method-of-Use Patents –
Protect the drug’s use for specific medical indications or populations.
6. Dosage Patents –
Cover specific dosages or dosing regimens proven to be effective.
7. Combination Patents –
Protect formulations combining two or more active ingredients.
💡 Note: A single FDA-approved drug can have multiple patents, each with its own expiration date.
What Is Exclusivity?
Exclusivity is a regulatory incentive granted to drug manufacturers after the approval of a new drug by the U.S. Food and Drug Administration (FDA). It prevents generic or biosimilar versions from being approved for a specified period of time—even if the drug is not patented.
FDA exclusivity is designed to reward innovation and encourage drug development, particularly for rare diseases or new therapeutic uses.
Key features of FDA exclusivity:
• Approved only at the time of FDA approval
• Protects the approved product from generic competition
• Applies to New Drug Applications (NDA) and Biologics License Applications (BLA)
• Can coexist with patents, but is independent of the USPTO.
How Long Does an Exclusivity Period Last?
The duration of exclusivity depends on the type of drug and approval pathway. Here are the common types and their respective durations:
| Type of Exclusivity | Duration | Description |
| New Chemical Entity (NCE) | 5 years | Granted for new active ingredients |
| Orphan Drug Exclusivity | 7 years | For drugs treating rare diseases (<200,000 patients) |
| Pediatric Exclusivity | +6 months | Added to existing patent or exclusivity |
| New Clinical Investigations | 3 years | For new uses or formulations requiring clinical data |
| Generic First Filer (Para IV) | 180 days | For first approved generic challenging a patent |
Important: Exclusivity only delays FDA approval of competing products. It does not stop others from developing or marketing a drug after the exclusivity expires—unless a valid patent still exists.
What Is the Difference Between Patent for pharmaceuticals and Exclusivity?
Though both serve to protect pharmaceutical innovations, patents and exclusivity differ in many ways:
| Aspect | Patent For Pharmaceuticals | Exclusivity |
| Authority | USPTO (U.S. Patent & Trademark Office) | FDA (Food & Drug Administration) |
| Purpose | Protects intellectual property | Encourages drug development |
| Granted When? | During drug development | Upon FDA approval |
| Duration | Usually 20 years from filing | 180 days to 7 years |
| Applies To | Molecules, formulations, processes | Approved drug products |
| Automatically Granted? | No, must be applied for | Yes, if conditions are met |
In short:
- Patent = Legal ownership
- Exclusivity = Regulatory protection
Why Does the Exclusivity Expire Before the Patent?
This is a very common situation.
Patents are filed early in the research and development process, before the drug reaches the market. On the other hand, exclusivity only starts after FDA approval.
So, if a patent is filed in 2010 and the drug is approved in 2020:
• The patent will expire in 2030
• But exclusivity can expire as early as 2025
Therefore, the duration of a patent is usually longer than exclusivity, unless the patent expires early or is declared invalid.
Patent Before Exclusivity? Exclusivity Before Patent?
Yes, both situations can occur:
• Pre-patent exclusivity: Common to almost all drugs. The company protects its invention before seeking FDA approval.
• Pre-patent exclusivity: Rare, but possible—especially in orphan drug cases or in pediatric studies where approval is granted before a patent is filed or published.
Why Does a Drug Have Only Patents and No Exclusivity?
Some drugs don’t qualify for exclusivity if they:
- Aren’t new chemical entities (NCEs)
- Don’t require new clinical investigations
- Are slight modifications of older drugs
- Don’t meet FDA exclusivity criteria
In such cases, companies rely entirely on patents to block competition and protect their products.
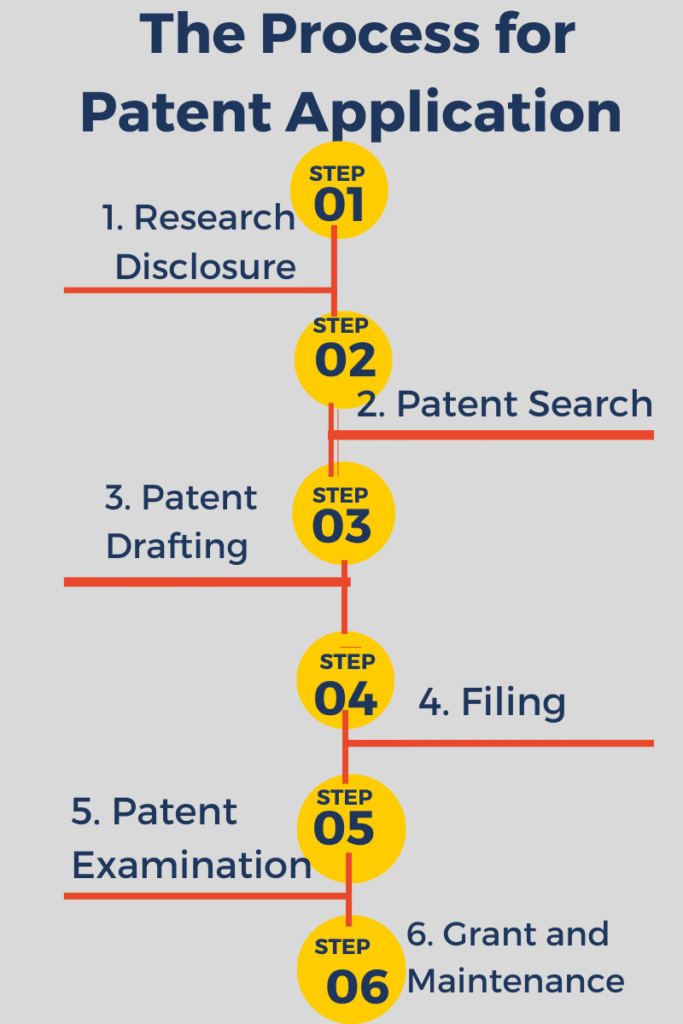
The Process for patent for pharmaceuticals Application in the U.S.
Here is a step-by-step analysis of how drug patents are filed in the US:
1. Research Disclosure
The research and development team prepares detailed data about the drug and its novelty.
2. Patent Search
Lawyers conduct an in-depth search using databases such as USPTO.gov and Google Patents.
3. Patent Drafting
Patent lawyers draft a complete application, including claims, chemical structures, and methods.
4. Filing
The application is submitted electronically through the USPTO electronic filing system.
5. Patent Examination
USPTO examiners review it for novelty, clarity, and utility.
6. Grant and Maintenance
Once approved, the patent is published. Maintenance fees must be paid periodically to keep it valid.
🕒 Duration: 2-4 years for grant approval.
Where Can I Find Patent and Exclusivity Regulations in the Code of Federal Regulations (C.F.R.)?
To explore the legal framework behind pharmaceutical patents and exclusivity, check these C.F.R. titles:
- 21 C.F.R. Part 314 — NDAs and exclusivity rules
- 21 C.F.R. Part 316 — Orphan drug regulations
- 37 C.F.R. Part 1 — Patent application and rules (USPTO)
Access these through the official Electronic Code of Federal Regulations: https://www.ecfr.gov
Final Thoughts :
Navigating the U.S. pharmaceutical market requires a deep understanding of both patents and exclusivity. Patents protect inventions, while FDA exclusivity encourages innovation by preventing generic competition. Whether you are a pharmacist, researcher, entrepreneur, or regulatory professional, understanding the scope and strategy of patents for medicines is essential to succeeding in 2025 and beyond.

