Blincyto Mechanism of Action: A Comprehensive Scientific and Clinical Overview
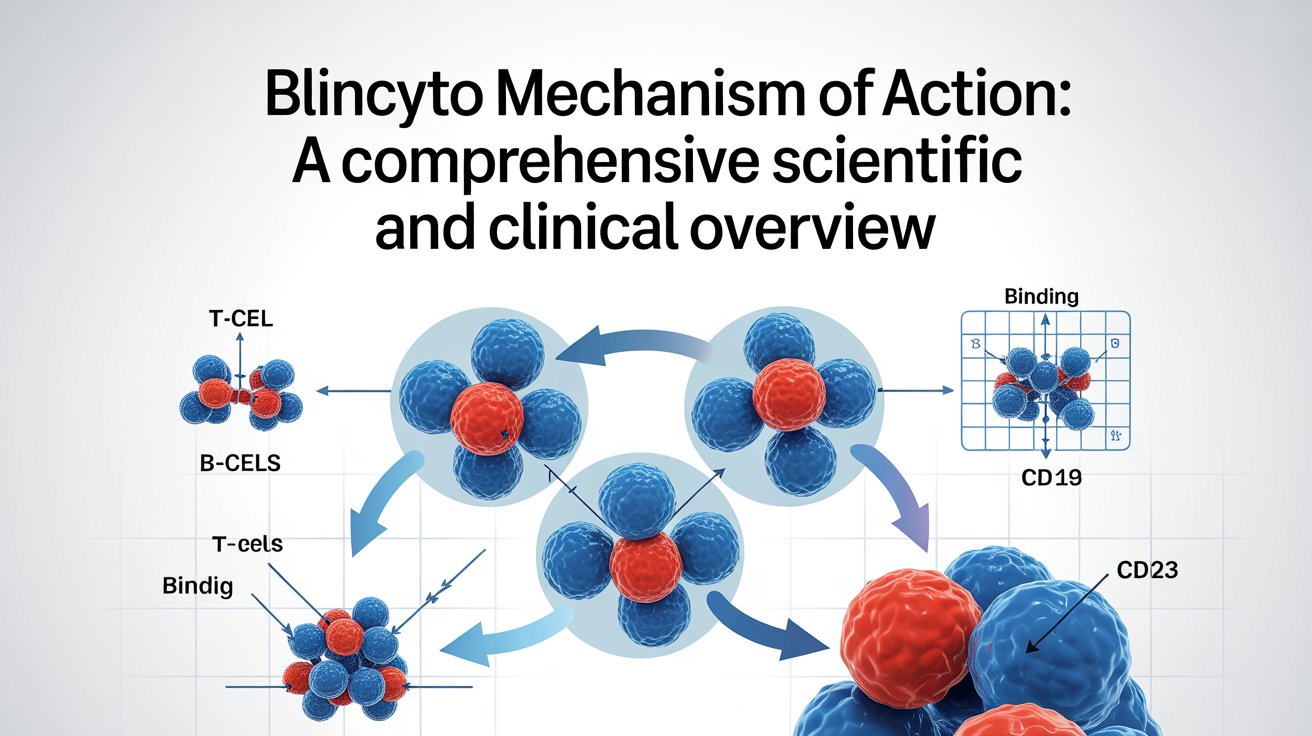
Introduction Immunotherapy has transformed the landscape of cancer treatment, especially in hematologic malignancies where traditional chemotherapy often falls short. One of the most groundbreaking innovations in this space is Blincyto (blinatumomab). The Blincyto mechanism of action represents a paradigm shift from non-specific cytotoxic therapy to precise immune-directed tumor killing. Blincyto is the first FDA-approved bispecific …