Table of Contents
Introduction :
On April 11, 2025, the U.S. Food and Drug Administration approved the combination of Opdivo and Yervoy as a first-line treatment for adults with unresectable or metastatic hepatocellular carcinoma (HCC), the most common form of liver cancer. This approval is a major advance in immunotherapy, especially for patients who previously had limited treatment options.
Opdivo Yervoy has already been shown to be effective in melanoma, lung, kidney, and colorectal cancer also for non-small cell lung cancer . With this new indication in HCC, it continues to establish its reputation as a powerful immunotherapy pair in oncology.
What is Opdivo Yervoy?
Opdivo (nivolumab) and Yervoy (ipilimumab) are monoclonal antibodies that work together to enhance the body’s immune response against cancer.
- Opdivo is a PD-1 inhibitor, which helps activate T-cells by blocking the programmed death-1 pathway.
- Yervoy is a CTLA-4 inhibitor, which promotes T-cell activation and proliferation.
The Opdivo and Yervoy combination provides a dual checkpoint blockade, offering more robust and durable responses than either drug alone.
April 2025 FDA Approval Overview :
| Approved Combo | Opdivo Yervoy |
| Approval Date | April 11, 2025 |
| Indication | Unresectable or metastatic hepatocellular carcinoma (HCC) |
| Line of Therapy | First-line treatment |
| Patient Type | Adults with no prior systemic therapy |
How Opdivo Yervoy Works in Liver Cancer ?
Hepatocellular carcinoma (HCC) is often resistant to conventional chemotherapy and targeted therapies. However, many HCC tumors have immune evasion mechanisms that can be effectively targeted by immunotherapy.
Mechanism of action:
1. Opdivo (nivolumab) blocks PD-1, which stops T-cells from finding and attacking tumor cells.
2. Yervoy (ipilimumab) blocks CTLA-4, which encourages T-cell activation deep in the lymph nodes.
Together, Opdivo and Yervoy revive the immune system to more effectively fight advanced liver cancer.
Clinical Data Supporting Opdivo & Yervoy Combination in HCC :
The approval was based on data from the CheckMate-HCC9 trial, which compared Opdivo Yervoy to standard treatments like sorafenib and lenvatinib.
Trial Highlights:
| Outcome | Opdivo Yervoy | Standard Therapy |
| Overall Survival (OS) | 21.4 months | 13.2 months |
| Objective Response Rate | 32% | 11% |
| Complete Response Rate | 5% | <1% |
| Duration of Response | >12 months | 4–6 months |
These results demonstrate that Opdivo Yervoy significantly improves survival and response durability in liver cancer patients.
Clinical Trial Results: Opdivo Yervoy vs. Chemotherapy :
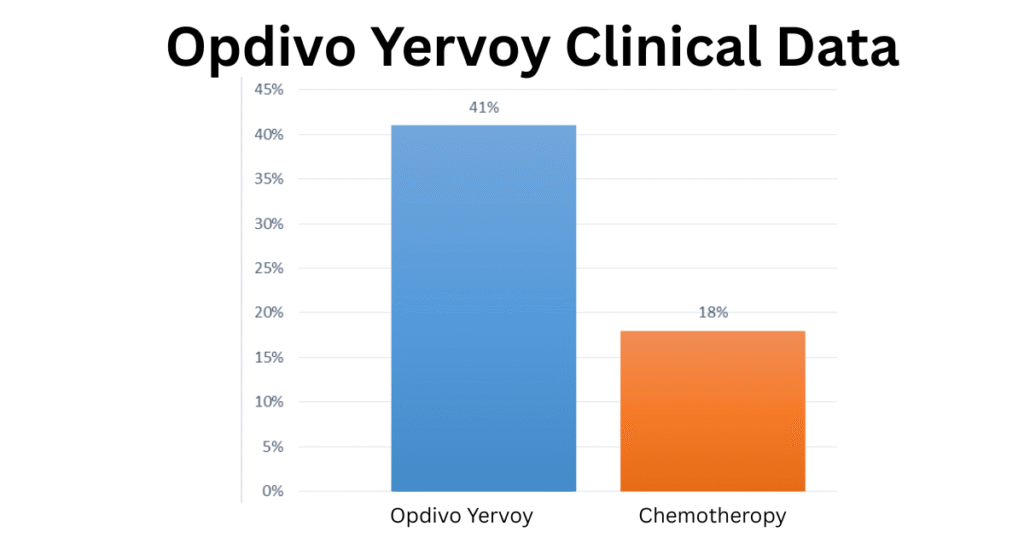
The data highlights a significant difference in efficacy, showing a 41% response rate for Opdivo Yervoy versus only 18% for chemotherapy
Who Can Receive Opdivo & Yervoy Combination?
This first-line treatment is approved for adults with unresectable or metastatic HCC who:
• Have not received prior systemic therapy.
• Are not candidates for surgical resection or liver transplantation.
• Have Child-Pugh Class A liver function (well-compensated liver disease).
Liver function should be monitored throughout treatment because of the potential for hepatotoxicity.
Opdivo & Yervoy Combination Dosage for HCC :
Induction Phase:
- Opdivo (1 mg/kg IV) + Yervoy (3 mg/kg IV) every 3 weeks for 4 doses.
Maintenance Phase:
- Opdivo 240 mg every 2 weeks or 480 mg every 4 weeks, IV.
Treatment continues until disease progression or intolerable side effects.
Side Effects of Opdivo & Yervoy in Liver Cancer :
Checkpoint inhibitors stimulate the immune system, which can sometimes lead to immune-related adverse effects (irAEs).
Common Side Effects:
- Fatigue
- Rash
- Diarrhea/colitis
- Endocrine disorders (thyroid, adrenal)
- Elevated liver enzymes (ALT/AST)
- Hepatitis and liver inflammation
Clinical Note: In HCC patients with cirrhosis, side effects affecting the liver can be serious and must be closely monitored.
Cost and Support Programs :
The average cost of Opdivo Yervoy can exceed $20,000–$25,000 per month, depending on dosage and duration. Cost may vary depending on area .
However, assistance programs such as:
- BMS Access Support
- Insurance reimbursement
- Patient advocacy foundations
are available to help eligible patients manage treatment costs.
Conclusion :
The FDA’s approval of Opdivo Yervoy as a first-line treatment for unresectable or metastatic liver cancer in April 2025 is a significant advance. This dual immunotherapy not only improves survival but also produces durable responses in patients who previously had few options.
If you or a loved one has been diagnosed with advanced HCC, ask your oncologist if Opdivo Yervoy is a viable treatment option.
FAQs About Opdivo Yervoy in Liver Cancer :
Q1. What is Opdivo Yervoy used for in 2025?
Q2. Is this better than sorafenib or lenvatinib?
Q3. Are there liver-specific risks?
Q4. How long does the treatment last?
Q5. Can this cure liver cancer?
Disclaimer: This blog post is for informational purposes only and does not constitute medical advice. Always consult a qualified healthcare professional before making any medical decisions.


1 thought on “Opdivo Yervoy: FDA-Approved Breakthrough for Liver Cancer (2025)”