Table of Contents
Targeted therapies have reshaped the treatment of hematological diseases. Mitapivat’s mechanism represents a paradigm shift in managing chronic hemolytic anemias, particularly Pyruvate Kinase Deficiency (PKD). Mitapivat (Pyrukynd) is a first-in-class, oral, small-molecule allosteric activator of the pyruvate kinase (PK) enzyme. Its U.S. Food and Drug Administration (FDA) approval was a significant milestone, offering a disease-modifying treatment where previously only supportive care was the standard of care.
This article delves into the intricate molecular biology of the mitapivat mechanism of action. We will explore the critical role of the PK enzyme in red blood cell (RBC) metabolism, how its deficiency leads to pathology, and the precise way mitapivat intervenes to restore cellular health. We will also cover the drug’s clinical journey, including its FDA approval, therapeutic indications, safety profile, and necessary considerations regarding drug interactions. Understanding the mitapivat mechanism of action is essential for grasping the full therapeutic potential of this innovative medication.
The Metabolic Context: Pyruvate Kinase Deficiency (PKD)
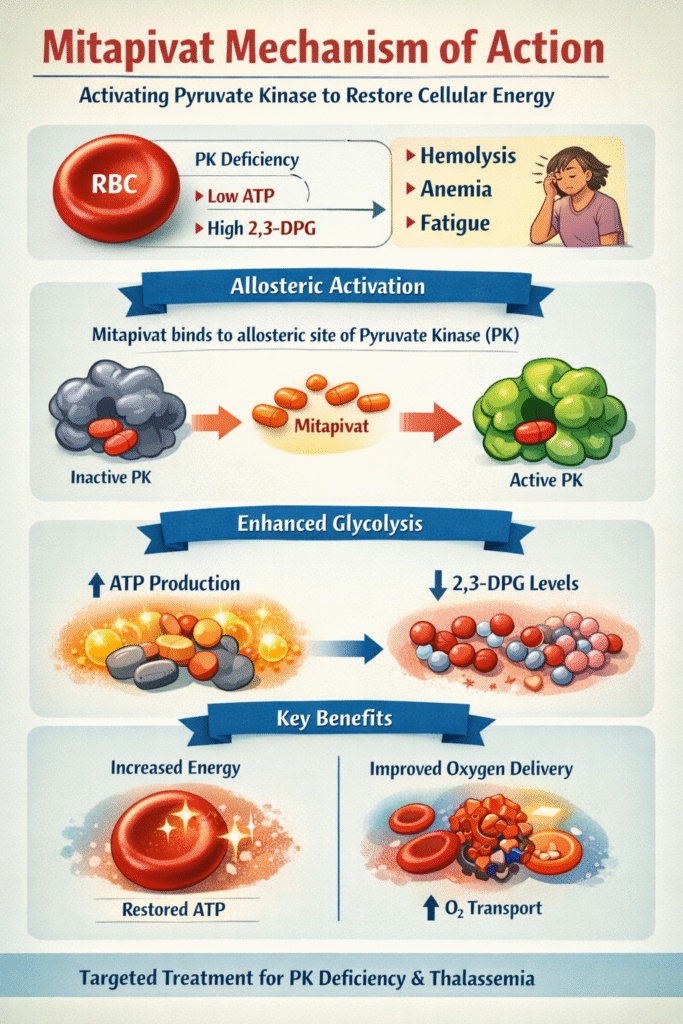
To fully appreciate the mitapivat mechanism of action, one must first understand the metabolic context of Pyruvate Kinase Deficiency. Red blood cells lack mitochondria and rely almost exclusively on anaerobic glycolysis for energy production. This pathway is critical for generating adenosine triphosphate (ATP), which maintains RBC membrane integrity and sustains cell lifespan.
Pyruvate kinase is the final, rate-limiting enzyme in glycolysis. It catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate, producing ATP in the process. In PKD, mutations in the PKLR gene lead to reduced or dysfunctional PK enzyme activity.
This defect leads to two major pathological consequences:
- ATP depletion, which compromises RBC structure and results in premature destruction and chronic hemolytic anemia
- Accumulation of upstream glycolytic intermediates such as 2,3-diphosphoglycerate (2,3-DPG), which decreases hemoglobin’s oxygen affinity and impairs tissue oxygen delivery
Patients experience fatigue, jaundice, splenomegaly, and often require lifelong blood transfusions. Mitapivat was developed to correct this fundamental metabolic failure.
The Core Mechanism: Mitapivat Mechanism of Action
The mitapivat mechanism of action is based on its function as an allosteric activator of pyruvate kinase. Rather than binding to the enzyme’s active site, mitapivat binds to a regulatory allosteric site, inducing a conformational change that stabilizes the enzyme in its active form.
Molecular and Cellular Effects
The mitapivat mechanism of action unfolds through the following steps:
- Allosteric binding stabilizes pyruvate kinase in its active R-state, counteracting destabilization caused by PKLR mutations
- Enhanced catalytic activity increases substrate affinity and raises maximum reaction velocity
- Restoration of glycolytic flux increases ATP generation, improving RBC membrane stability and lifespan
- Reduced accumulation of 2,3-DPG improves hemoglobin oxygen-binding capacity and tissue oxygen delivery
These combined effects directly address the bioenergetic failure responsible for hemolysis. Clinical trials have demonstrated consistent efficacy across multiple PKD genotypes, confirming the broad effectiveness of the mitapivat mechanism of action.
Beyond PKD: Mitapivat in Thalassemia
The mitapivat mechanism of action also benefits patients with alpha- and beta-thalassemia. In these conditions, chronic oxidative stress and ineffective erythropoiesis contribute to RBC destruction.
By improving ATP availability and metabolic efficiency, mitapivat enhances erythrocyte resilience and reduces ineffective erythropoiesis. Clinical trials have shown improved hemoglobin levels and reduced transfusion requirements, supporting the expanded therapeutic role of the mitapivat mechanism of action.
Clinical Milestones: FDA Approval and Indications
FDA Approval for Pyruvate Kinase Deficiency
Mitapivat received FDA approval on February 17, 2022, for the treatment of hemolytic anemia in adults with PK deficiency. This approval was supported by the Phase 3 ACTIVATE and ACTIVATE-T trials, which demonstrated sustained hemoglobin improvement and reduced transfusion dependency.
Expanded Indications
In December 2025, the FDA approved mitapivat (AQVESME) for the treatment of anemia in adults with alpha- or beta-thalassemia. Data from the ENERGIZE and ENERGIZE-T trials confirmed that the metabolic benefits of the mitapivat mechanism of action translate into meaningful clinical outcomes in thalassemia patients.
Safety Profile and Side Effects
Clinical studies show that mitapivat is generally well tolerated.
Common Adverse Reactions
Most commonly reported side effects include:
- Nausea, vomiting, and diarrhea
- Back pain and joint pain
- Elevated uric acid levels
- Hormonal changes such as decreased estrone and estradiol in males, typically without symptoms
Serious Warnings and Precautions
Abrupt discontinuation may cause acute hemolysis due to sudden loss of ATP support. Gradual tapering is required.
Hepatocellular injury has been observed, necessitating routine liver function monitoring, particularly during the first six months of therapy.
Navigating Drug Interactions
Mitapivat is primarily metabolized by CYP3A and can induce or inhibit several drug-metabolizing enzymes and transporters.
| Interacting Drug Class | Effect | Recommendation |
|---|---|---|
| Strong CYP3A inhibitors | Increased exposure | Avoid use |
| Strong CYP3A inducers | Decreased exposure | Avoid use |
| Moderate CYP3A inhibitors | Increased exposure | Limit dose |
| Moderate CYP3A inducers | Decreased exposure | Adjust or avoid |
Mitapivat may reduce the effectiveness of hormonal contraceptives and other narrow therapeutic index drugs, requiring careful medication review prior to initiation.
Conclusion
The mitapivat mechanism of action represents a transformative advance in hematology by directly correcting metabolic dysfunction in red blood cells. Through targeted activation of pyruvate kinase, mitapivat restores ATP production, reduces hemolysis, and improves oxygen delivery.
Its FDA approval and expanded indications confirm its role as a cornerstone therapy for PK deficiency and thalassemia. With appropriate monitoring and drug interaction management, mitapivat offers a safe and effective disease-modifying option for patients with chronic hemolytic anemia.

