Table of Contents
Introduction
Prostate cancer is one of the most commonly diagnosed cancers in men worldwide. Accurate diagnosis and staging are crucial for choosing the right treatment plan and improving patient outcomes. Conventional imaging methods often fall short of detecting small or early lesions, especially in cases of recurrence.
This is where Gozellix® comes in. After radiolabeling with gallium-68 (68Ga), Gozellix is used for positron emission tomography (PET) imaging to identify PSMA-positive lesions in men with prostate cancer. Its primary role is to provide high-precision imaging in:
• Patients with suspected metastases who are candidates for definitive therapy.
• Patients with suspected recurrence based on elevated serum PSA levels.
In this blog, we will explore Gozellix in detail – its mechanism of action, clinical applications, safety profile, advantages over conventional imaging, and its future potential in prostate cancer management.
Understanding PSMA-targeted PET imaging
Gozellix® is a radiopharmaceutical agent specifically developed for prostate cancer imaging. It works by binding to prostate-specific membrane antigen (PSMA), a protein highly expressed on prostate cancer cells. When labeled with gallium-68, it allows PET scanners to detect cancerous lesions with high accuracy.
Key features
• Target-specific: Binds to PSMA receptors on prostate cancer cells.
• High sensitivity: Detects lesions that may be missed with conventional CT or MRI.
• Non-invasive: Provides detailed internal imaging without surgery.
• Versatile use: Applicable in both initial staging and repeat evaluation.
The Science Behind radiolabeled Gallium-68 agent
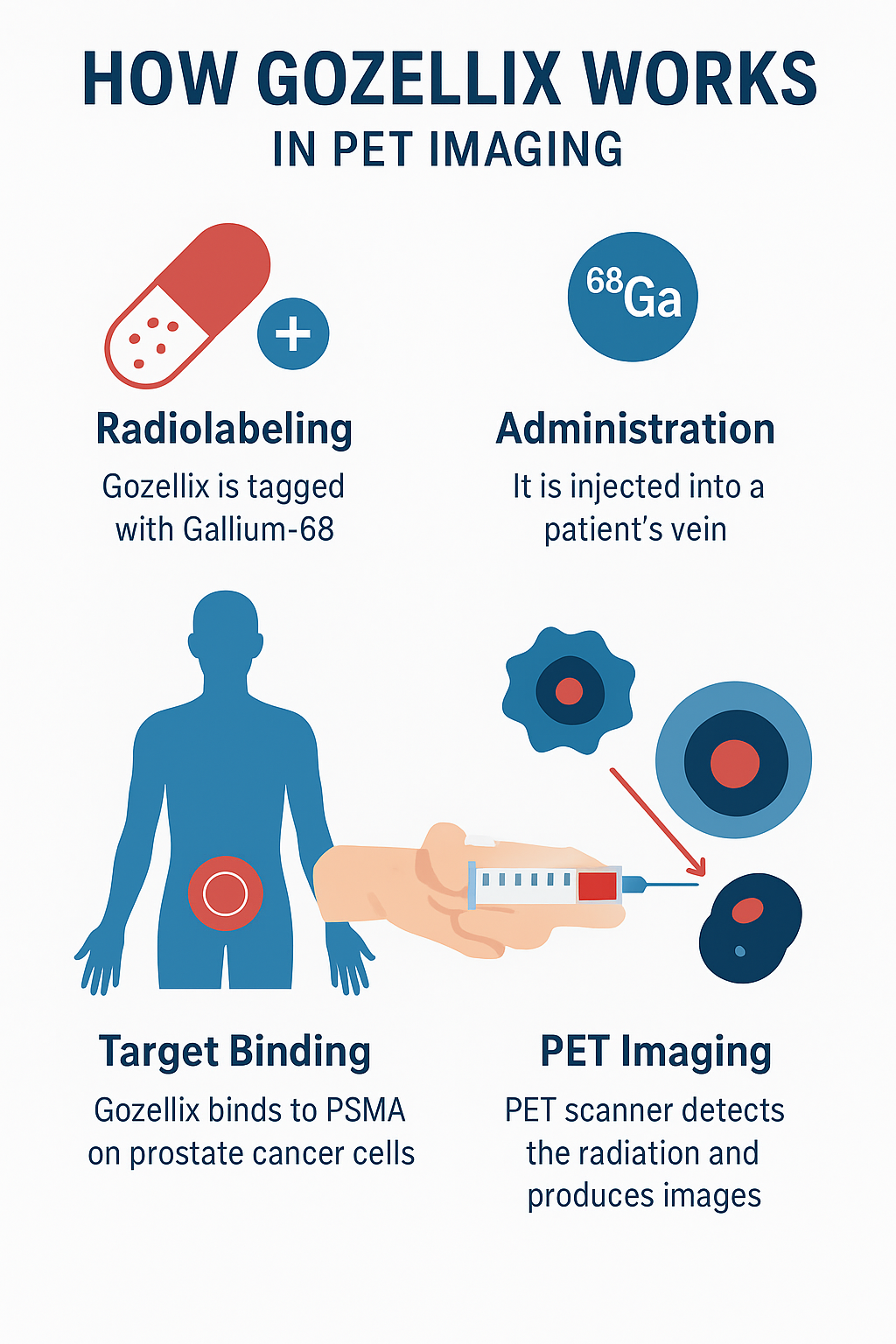
The effectiveness of Gozellix depends on radiolabeling with Gallium-68 (68Ga). Here’s how it works:
- Radiolabeling process – Gozellix is tagged with Gallium-68, a positron-emitting isotope.
- Administration – The radiolabeled drug is injected intravenously.
- Target binding – It circulates in the body and binds to PSMA receptors on prostate cancer cells.
- PET imaging – PET scanners detect the emitted radiation, producing high-resolution images.
This targeted approach makes Gozellix a reliable imaging tool for detecting both localized and metastatic prostate cancer.
Clinical Indications
Gozellix is indicated for use in men with prostate cancer in two specific scenarios:
1. Suspected Metastasis in Initial Diagnosis
- Patients with newly diagnosed prostate cancer who are being considered for initial definitive therapy (such as surgery or radiation).
- Helps determine if cancer has spread beyond the prostate gland.
- Guides treatment decisions, e.g., whether localized therapy is sufficient or systemic treatment is required.
2. Suspected Recurrence Based on PSA Levels
- Patients who previously underwent treatment (surgery, radiation, hormone therapy) but show elevated serum PSA levels.
- Identifies the location of recurrence — whether it is local, regional, or distant metastasis.
- Helps oncologists decide the next line of treatment.
Why Gozellix is a Game-Changer
1. Higher Accuracy Compared to Conventional Imaging
Standard imaging modalities like CT or bone scans often miss small metastatic lesions. PET imaging provides:
- Better sensitivity in detecting small lesions.
- Improved specificity, reducing false positives.
2. Early Detection of Recurrence
Prostate cancer recurrence can be difficult to locate. PSMA-targeted PET imaging agent detects recurrent disease at an early stage, even when PSA levels are only mildly elevated.
3. Personalized Treatment Planning
By mapping cancer spread with accuracy, radiolabeled Gallium-68 agent helps tailor treatment strategies — avoiding overtreatment or undertreatment.
4. Non-Invasive and Safe
Patients undergo a quick injection followed by a PET scan, avoiding invasive procedures.
Clinical Evidence Supporting Gozellix
Several studies have shown the clinical utility of PSMA PET imaging agents like Gozellix:
- Improved staging accuracy – Identifies metastases not seen in CT or MRI scans.
- Better recurrence localization – Detects recurrent lesions in nearly 30–40% more patients compared to standard imaging.
- Impact on treatment decisions – Research indicates that up to 50% of patients had changes in treatment planning based on PET imaging results with PSMA-targeted radiopharmaceuticals.
Safety Profile
Radiolabeled Gallium-68 agent is generally well tolerated. As with any radiopharmaceutical, risks are minimal but can include:
- Mild injection site reactions (redness, swelling).
- Rare allergic responses.
- Low radiation exposure (within safe diagnostic limits).
The benefits of accurate cancer detection far outweigh these minimal risks
Comparison: Gozellix vs. Conventional Imaging
| Feature | Conventional Imaging (CT, MRI, Bone Scan) | Gozellix PET Imaging |
| Sensitivity | Moderate | High |
| Specificity | Variable | High |
| Detection of micro-metastases | Poor | Excellent |
| Radiation dose | Variable | Low & controlled |
| Impact on treatment decisions | Limited | Significant |
Future Outlook of Radiolabeled Gallium-68 agent
As the field of nuclear medicine and molecular imaging advances, agents will continue to play a pivotal role in:
- Early prostate cancer detection.
- Monitoring treatment response.
- Guiding novel therapies such as PSMA-targeted radionuclide therapy.
With ongoing clinical trials and global adoption, PET radiotracer
is expected to become a standard of care in prostate cancer management.
Patient Experience: What to Expect During a Gozellix PET Scan
- Preparation – Minimal preparation required, except for standard PET scan guidelines.
- Injection – Radiolabeled Gozellix is administered intravenously.
- Waiting period – Short uptake time for the agent to bind to cancer cells.
- PET scan – Patients undergo imaging, usually completed within 30–45 minutes.
- Post-scan – Normal activities can be resumed soon after, as radiation exposure is minimal.
Conclusion
Prostate cancer management relies heavily on accurate imaging to guide treatment strategies. Gozellix®, radiolabeled with gallium-68, provides a state-of-the-art diagnostic solution for the detection of PSMA-positive lesions in men with prostate cancer. Whether in the early stages of newly diagnosed cases or in identifying recurrences, PSMA-targeted PET imaging
ensures greater accuracy, safety, and reliability compared to traditional methods.
As precision medicine advances, agents like Gozellix will shape the future of prostate cancer diagnosis and therapy planning, improving patient outcomes worldwide.
Frequently Asked Questions (FAQs)
1. What is Gozellix used for?
2. How accurate is Gozellix compared to MRI or CT?
3. Is PSMA-targeted PET imaging agent
safe?
4. Can Gozellix detect recurrence early?
5. Who should undergo a prostate cancer imaging tracer
PET scan?
Disclaimer
This article is for informational and educational purposes only. It is not intended to provide medical advice, diagnosis, or treatment. Always consult a qualified healthcare professional before making any decisions about your health, diagnosis, or treatment options.