Brand name: Qfitlia
Generic name: fitusiran
Dosage form: Injection
Drug class : Antithrombin-targeting siRNA
Table of Contents
Introduction :
In March 2025, a historic breakthrough was made in the world of rare diseases – the US FDA officially approved Fitusiran approval for the treatment of hemophilia A and B, with and without inhibitors. The approval was long-awaited due to the drug’s revolutionary RNA interference (RNAi) technology, developed by Alnylam Pharmaceuticals and co-developed by Sanofi.
The Fitusiran approval brings a transformative option to thousands of patients who have long relied on repeated intravenous factor infusions or bypassing agents. This once-monthly subcutaneous therapy has the potential to significantly reduce bleeding episodes, improve patients’ quality of life, and simplify disease management.
What is Fitusiran?
Fitusiran is a small interfering RNA (siRNA) therapeutic drug designed to treat patients with hemophilia A or B, regardless of the presence of inhibitors. Unlike traditional treatments that aim to replace missing clotting factors, Fitusiran works by targeting the liver’s production of antithrombin (AT) – a natural anticoagulant protein.
- Brand Name: Qfitlia
- Approval Date: 28 March 2025 (FDA)
- Route of Administration: Subcutaneous injection (once monthly)
- Indication: Prophylactic treatment of Hemophilia A and B, with or without inhibitors
What is hemophilia A and B ?
Hemophilia A and B are rare, inherited bleeding disorders that cause the body to be unable to form proper blood clots. They are caused by a deficiency of certain clotting factors that are needed for normal blood clotting. In hemophilia A, there is a deficiency or complete absence of clotting factor VIII (8), while hemophilia B is caused by a deficiency or absence of clotting factor IX (9). Both conditions are X-linked recessive disorders, meaning they predominantly affect males, while females are usually carriers and may have milder symptoms in some cases.
The symptoms of both types of hemophilia are very similar and include easy bruising, prolonged bleeding after injury or surgery, spontaneous internal bleeding (especially into joints and muscles), and excessive bleeding after dental procedures. The severity of the condition can vary depending on the amount of clotting factor in the blood. Severe forms of hemophilia can cause life-threatening bleeding or long-term damage to joints due to repeated bleeding.
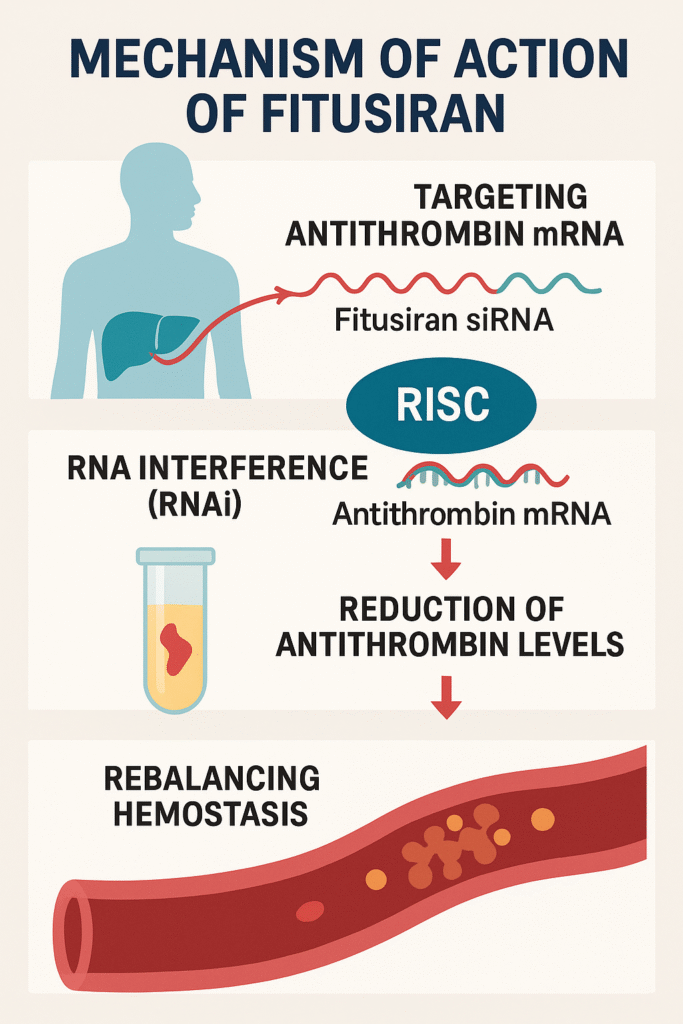
Mechanism of Action: Targeting Antithrombin with siRNA :
Fitusiran is a small interfering RNA (siRNA) therapeutic that works through RNA interference (RNAi) to reduce the production of antithrombin (AT) – a natural anticoagulant protein that is produced primarily in the liver.
1. Targeting antithrombin mRNA:
Fitusiran is specifically designed to bind to the messenger RNA (mRNA) that codes for antithrombin (AT) in liver cells (hepatocytes).
2. RNA interference (RNAi):
Once in liver cells, Fitusiran uses the RNA-induced silencing complex (RISC) to degrade antithrombin mRNA, thereby preventing its conversion to antithrombin protein.
3. Reducing antithrombin levels:
This reduces the plasma levels of antithrombin, which reduces its natural inhibitory effect on thrombin and other clotting enzymes.
4. Rebalancing hemostasis:
By reducing antithrombin, Fitusiran increases thrombin generation, which helps clot formation. This compensates for the deficiency of blood clotting factors (factor VIII in hemophilia A or factor IX in hemophilia B).
5. Effective in patients with or without inhibitors:
The action of Fitusiran is not dependent on factor replacement, making it particularly beneficial for patients who have developed inhibitors to factor VIII or IX, a major complication of conventional therapy.
Clinical Trials Supporting Fitusiran Approval :
🔬 ATLAS-INH Study (Inhibitor Population)
- Participants: Hemophilia A/B with inhibitors
- Result: 89.9% reduction in annualized bleeding rate (ABR) compared to on-demand treatment
- Safety: Managed thrombotic risk through patient selection and dose titration
🧪 ATLAS-A/B Study (Non-inhibitor Population)
- Participants: Hemophilia A/B without inhibitors
- Result: 85.1% ABR reduction vs on-demand therapy
- Conclusion: Strong efficacy across both hemophilia types
🔍 ATLAS-PPX Study (Prophylactic Comparison)
- Compared to: Standard prophylactic factor therapy
- Result: Fitusiran showed statistically superior reduction in bleeding rates
These clinical trials formed the foundation for the FDA fitusiran approval in March 2025.
Benefits of Fitusiran Approval Over Traditional Therapies :
| Feature | Fitusiran | Traditional Therapy |
| Administration | Subcutaneous (monthly) | Intravenous (2-3x/week) |
| Efficacy | ~90% bleed reduction | ~60–70% bleed reduction |
| Inhibitor Patients | Effective | Limited |
| Patient Burden | Low | High |
| Mechanism | siRNA (targets AT) | Clotting factor replacement |
Global Regulatory Landscape After March 2025 :
Following the U.S. approval on 28 March 2025, regulatory momentum is building worldwide:
- 🇪🇺 EMA Approval: Expected in Q3 2025
- 🇨🇦 Health Canada: Application under accelerated review
- 🇯🇵 PMDA (Japan): Phase 3 results submitted
- 🇦🇺 TGA (Australia): Conditional approval anticipated by end of 2025
The fitusiran approval is driving global excitement in the hemophilia community, with widespread access expected by mid-2026.
Safety Profile and Side Effects :
Common Side Effects:
- Injection site pain or redness
- Headache
- Nausea
- Elevated liver enzymes
Serious Adverse Events:
- Thromboembolic events (rare): Pulmonary embolism, deep vein thrombosis
- Liver function abnormalities
- Low fibrinogen levels
Risk Mitigation Strategies:
- Regular liver function tests
- Monitoring for thrombotic events
- Tailored dosing in high-risk patients
Despite the risks, clinical benefits outweigh concerns when the drug is properly monitored.
Real-World Patient Impact :
Patients using Fitusiran in expanded access programs and trials report:
- Drastically fewer spontaneous bleeds
- Improved mobility and daily activity
- Better mental health and reduced fear of bleeding
- Fewer hospital visits and lower caregiver stress
For inhibitor patients, Fitusiran offers a new lease on life, reducing reliance on costly bypassing agents.
Future of Fitusiran and RNAi Therapies :
- Pediatric Trials: Currently underway to extend use in children
- Real-World Evidence Studies: Ongoing post-marketing surveillance to track long-term safety
- Pipeline Expansion: Potential use in other clotting disorders (e.g., antithrombin deficiencies)
- Combination Therapy Trials: May pair with gene therapies for enhanced results
The fitusiran approval is just the beginning of a broader RNAi revolution in rare disease treatment.
Conclusion :
The Fitusiran approval on March 28, 2025, is a monumental event in hemophilia care. For patients around the world, it means less bleeding, simpler treatment regimens, and improved quality of life. With a novel siRNA mechanism and broad efficacy, Fitusiran is paving the way for a new generation of precision therapies in rare diseases. If you or someone you know has hemophilia, now is the time to explore how Fitusiran could change the future of care.
Frequently Asked Questions (FAQs) :
1. When was Fitusiran approved?
2. How does Fitusiran work?
3. Is Fitusiran suitable for inhibitor patients?
4. How is Fitusiran administered?
5. Are there any serious side effects?
6. Fitusiran can cause Weight loss ?
Disclaimer: This blog post is for informational purposes only and does not constitute medical advice. Always consult a qualified healthcare professional before making any medical decisions.

