Table of Contents
Introduction
The healthcare landscape in 2026 continues to evolve rapidly, especially in oncology where innovation in both treatment and supportive care remains critical. Among the major regulatory milestones this year is the Filkri biosimilar Approval, a development that holds significant implications for cancer patients undergoing chemotherapy. Supportive oncology care is no longer considered secondary; it is now a cornerstone of comprehensive cancer management. Managing anemia, preventing treatment interruptions, and improving quality of life are just as important as targeting tumors directly.
The Filkri biosimilar Approval represents a strategic advancement in managing chemotherapy-induced anemia. As cancer treatments become more aggressive and personalized, patients increasingly rely on supportive agents to maintain strength and tolerate therapy. This approval introduces a cost-effective and clinically comparable option to existing biologics, expanding patient access and optimizing healthcare resources.
In this comprehensive guide, we will explore what the Filkri biosimilar Approval means for supportive oncology care, its mechanism of action, safety profile, drug interactions, FDA pathway, economic implications, and its broader relevance when compared to chronic conditions such as hypertrophic cardiomyopathy.
Understanding Supportive Oncology Care in 2026
Supportive oncology care focuses on minimizing the physical and psychological burden of cancer therapy. While chemotherapy, immunotherapy, and targeted therapies attack cancer cells, they often damage healthy tissues as well. Bone marrow suppression is one of the most common adverse effects, leading to anemia, neutropenia, and thrombocytopenia.
Anemia, in particular, affects a large proportion of chemotherapy patients. Fatigue, dizziness, shortness of breath, and reduced physical endurance can significantly compromise daily functioning. Before biosimilars, supportive care relied heavily on originator biologics and blood transfusions. The Filkri biosimilar Approval introduces a modern alternative that strengthens the supportive care arsenal.
With the Filkri biosimilar Approval, oncology teams can integrate an additional erythropoiesis-stimulating agent (ESA) option into treatment protocols. This approval signals increased competition in the biologics market, potentially lowering costs and broadening accessibility.
What Is Filkri?
Filkri is a biosimilar version of a reference erythropoietin-based biologic used in the management of chemotherapy-induced anemia. Biosimilars are not generic drugs; they are highly similar biological products developed after the patent expiration of a reference biologic.
The Filkri biosimilar Approval confirms that the product demonstrates no clinically meaningful differences in safety, purity, and potency compared to its reference product. Analytical studies, pharmacokinetic comparisons, and clinical trials form the backbone of this regulatory decision.
Unlike small-molecule drugs, biologics are complex proteins produced in living cells. Therefore, achieving regulatory clearance requires extensive structural and functional comparison. The Filkri biosimilar Approval reflects successful demonstration of biosimilarity through rigorous evaluation.
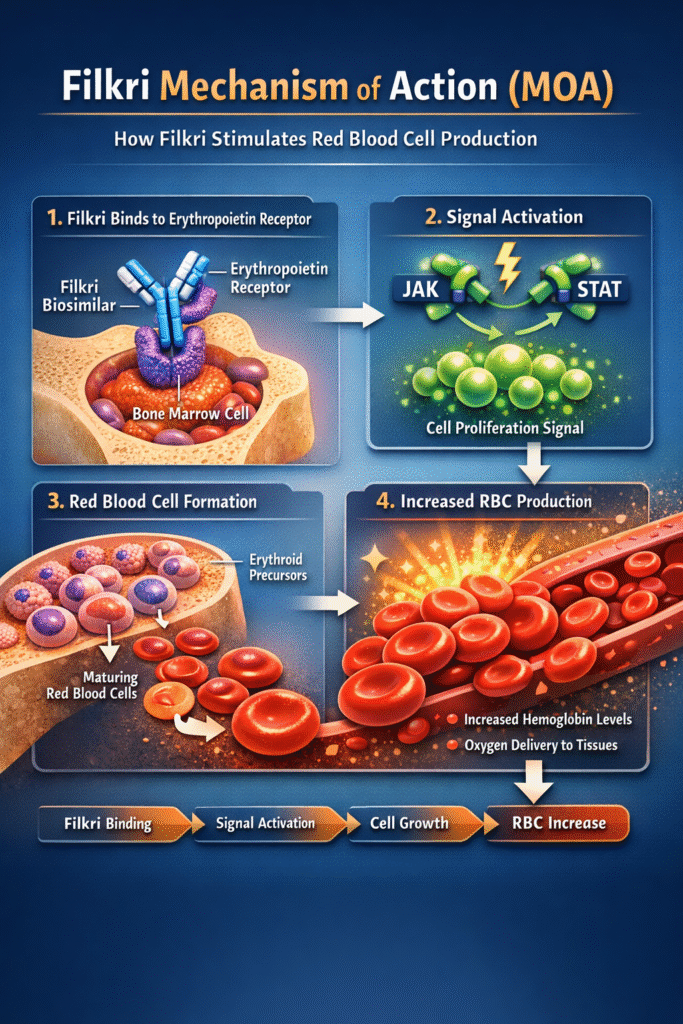
Mechanism of Action (MOA)
The therapeutic effectiveness behind the Filkri biosimilar Approval lies in its ability to mimic endogenous erythropoietin. Erythropoietin is a glycoprotein hormone produced primarily by the kidneys in response to hypoxia.
Step-by-Step Mechanism
- Receptor Binding
Filkri binds to erythropoietin receptors located on erythroid progenitor cells in the bone marrow. - Signal Activation
Binding activates intracellular signaling pathways, particularly the JAK2/STAT pathway. - Cell Proliferation and Differentiation
These signals stimulate red blood cell precursor cells to proliferate and mature. - Increased Red Blood Cell Production
The final outcome is elevated hemoglobin levels and improved oxygen delivery to tissues.
The Filkri biosimilar Approval confirms identical receptor interaction and biological activity compared to the reference biologic. This ensures predictable clinical outcomes.
FDA Approval Pathway and Regulatory Standards
The Filkri biosimilar Approval was granted under the biosimilar regulatory pathway, which requires:
- Comprehensive analytical similarity studies
- Animal toxicology assessments
- Comparative clinical studies
- Pharmacokinetic and pharmacodynamic evaluations
- Immunogenicity analysis
Regulatory authorities carefully assess structural integrity, glycosylation patterns, and biological function. The Filkri biosimilar Approval indicates that these stringent criteria were successfully met.
Unlike novel drug approvals that require extensive efficacy trials, biosimilar approvals focus on demonstrating equivalence rather than independent efficacy. This efficient pathway reduces development costs and ultimately benefits healthcare systems.
Clinical Benefits in Supportive Oncology
1. Reduction in Transfusion Dependency
Blood transfusions carry risks such as transfusion reactions and infections. With the Filkri biosimilar Approval, patients have access to a therapy that stimulates endogenous red blood cell production, potentially reducing transfusion frequency.
2. Improved Treatment Continuity
Chemotherapy delays often occur due to severe anemia. The Filkri biosimilar Approval supports proactive anemia management, allowing patients to remain on schedule.
3. Enhanced Quality of Life
Fatigue is one of the most debilitating chemotherapy side effects. By restoring hemoglobin levels, therapies authorized under the Filkri biosimilar Approval help improve physical functioning and overall well-being.
4. Expanded Access Through Cost Efficiency
One of the major advantages following the Filkri biosimilar Approval is potential price reduction compared to originator biologics. This may improve treatment accessibility globally.
Side Effects and Safety Profile
While effective, therapies included under the Filkri biosimilar Approval require careful monitoring.
Common Side Effects
- Headache
- Injection site pain
- Fatigue
- Nausea
- Joint discomfort
Serious Risks
- Hypertension
- Thromboembolic events
- Rare cases of pure red cell aplasia
- Potential tumor progression concerns in specific cancers
The safety data reviewed during the Filkri biosimilar Approval process showed comparable adverse event rates to the reference product.
Monitoring recommendations include:
- Regular hemoglobin checks
- Blood pressure assessment
- Evaluation for clotting risk
Drug Interactions
Direct pharmacokinetic interactions are minimal because Filkri works via receptor stimulation rather than liver enzyme modulation. However, the Filkri biosimilar Approval includes guidance on clinical considerations.
Iron Supplements
Iron deficiency can reduce ESA effectiveness. Iron therapy may be necessary alongside Filkri.
Anticoagulants
Because of clotting risk, patients on anticoagulants should be monitored closely.
Antihypertensive Medications
Blood pressure elevation may require dose adjustments of antihypertensive drugs.
Chemotherapy Timing
Coordination with oncology regimens ensures optimal results under the Filkri biosimilar Approval framework.
Economic Impact on Healthcare Systems
The introduction of biosimilars often reshapes pharmaceutical economics. The Filkri biosimilar Approval is expected to:
- Increase market competition
- Lower overall ESA treatment costs
- Expand formulary inclusion
- Reduce long-term healthcare spending
Healthcare systems under financial pressure may particularly benefit from the cost efficiency following the Filkri biosimilar Approval.
Real-World Application and Post-Marketing Surveillance
Post-approval monitoring remains critical. After the Filkri biosimilar Approval, pharmacovigilance systems track:
- Immunogenic responses
- Long-term safety outcomes
- Patient adherence
- Comparative effectiveness
Real-world evidence further validates the clinical reliability established during the Filkri biosimilar Approval process.
Comparison with Hypertrophic Cardiomyopathy Therapies
Hypertrophic cardiomyopathy (HCM) is a chronic cardiac disorder involving thickened heart muscle and impaired blood flow. Although unrelated to anemia management, comparing therapeutic strategies provides clinical perspective.
HCM treatment typically involves:
- Beta blockers
- Calcium channel blockers
- Myosin inhibitors
- Septal reduction procedures
Unlike anemia therapy under the Filkri biosimilar Approval, HCM drugs target cardiac muscle contractility and outflow obstruction.
Both areas emphasize symptom control and improved quality of life. The difference lies in biological targets: erythropoietin receptors in oncology supportive care versus myocardial contractile pathways in HCM.
The Filkri biosimilar Approval reflects progress in biologic supportive therapy, while cardiovascular drug innovation represents advancement in structural heart disease management.
Future Outlook for Biosimilars in Oncology
The Filkri biosimilar Approval may serve as a model for future supportive oncology biosimilars. As patents expire, more biologic categories may see biosimilar competition.
Trends likely include:
- Greater interchangeability acceptance
- Expanded payer coverage
- Increased physician confidence
- Broader global access
The oncology ecosystem is steadily embracing biosimilars, and the Filkri biosimilar Approval is part of this transformative movement.
Clinical Integration Strategy
To optimize outcomes after the Filkri biosimilar Approval, healthcare teams should:
- Educate patients about anemia management.
- Establish monitoring protocols.
- Coordinate multidisciplinary oncology teams.
- Document hemoglobin targets clearly.
- Maintain pharmacovigilance reporting systems.
Proper integration maximizes therapeutic benefits under the Filkri biosimilar Approval.
Conclusion
The Filkri biosimilar Approval marks a significant advancement in supportive oncology care in 2026. By offering a clinically comparable and potentially more affordable alternative to established biologics, this approval strengthens anemia management strategies for cancer patients.
From mechanism of action to safety monitoring, economic impact to healthcare accessibility, the implications of the Filkri biosimilar Approval extend beyond a single drug authorization. It represents progress in biologic innovation, regulatory science, and patient-centered care.
As oncology continues to evolve, supportive therapies remain essential pillars of treatment. The future of cancer care depends not only on tumor-targeting drugs but also on supportive solutions like those enabled through the Filkri biosimilar Approval.

