Table of Contents
Introduction
Effective control of bleeding is a cornerstone of modern medical care, particularly in patients with inherited or acquired coagulation disorders. Among all coagulation factors, fibrinogen plays a foundational role because it is essential for the final formation of a stable blood clot. Fibrinogen human-chmt (fesilty) is a plasma-derived fibrinogen concentrate developed to address this critical need in patients who are unable to produce adequate fibrinogen on their own.
Fibrinogen human-chmt is used primarily in patients with congenital fibrinogen deficiency, a rare but potentially life-threatening condition characterized by spontaneous bleeding, excessive surgical bleeding, and poor wound healing. The availability of fibrinogen human-chmt represents a major advancement over traditional plasma products by offering a purified, standardized, and rapidly acting fibrinogen replacement therapy.
This expanded article provides a detailed clinical and pharmacological overview of fibrinogen human-chmt, explaining how it works, when it is used, its FDA approval background, safety considerations, and its growing role in modern hemostatic management.
Understanding Fibrinogen and Its Role in Hemostasis
Fibrinogen is a large glycoprotein synthesized in the liver and released into the bloodstream, where it circulates at relatively high concentrations compared to other clotting factors. It serves two critical roles in hemostasis:
- Platelet Aggregation – Fibrinogen binds to activated platelets, forming bridges that allow platelets to clump together at the site of vascular injury.
- Fibrin Clot Formation – Fibrinogen is converted into fibrin strands that form the structural framework of a blood clot.
In the absence of adequate fibrinogen, clot formation is weak, unstable, or completely absent. Fibrinogen human-chmt directly replaces this missing protein, restoring both platelet function and clot integrity.
What Is Fibrinogen human-chmt ?
Fibrinogen human-chmt is a sterile, lyophilized concentrate of human fibrinogen (coagulation factor I) derived from pooled human plasma. It undergoes extensive purification and viral inactivation steps to enhance safety. Once reconstituted, it is administered intravenously.
Unlike cryoprecipitate, which contains variable amounts of fibrinogen and other clotting proteins, fesilty provides a precise and predictable dose of fibrinogen. This allows clinicians to achieve targeted fibrinogen levels quickly and reliably.
FDA Approval –
A major milestone in the clinical development of this therapy occurred when fibrinogen human-chmt was approved by the FDA on December 16, 2025.
Approved Indication
The FDA approval covers:
- Treatment of acute bleeding episodes in adult and pediatric patients
- Patients with congenital fibrinogen deficiency, including afibrinogenemia and hypofibrinogenemia
This approval confirms that fibrinogen human-chmt meets regulatory standards for safety, efficacy, and manufacturing quality, making it a trusted therapeutic option for rare bleeding disorders.
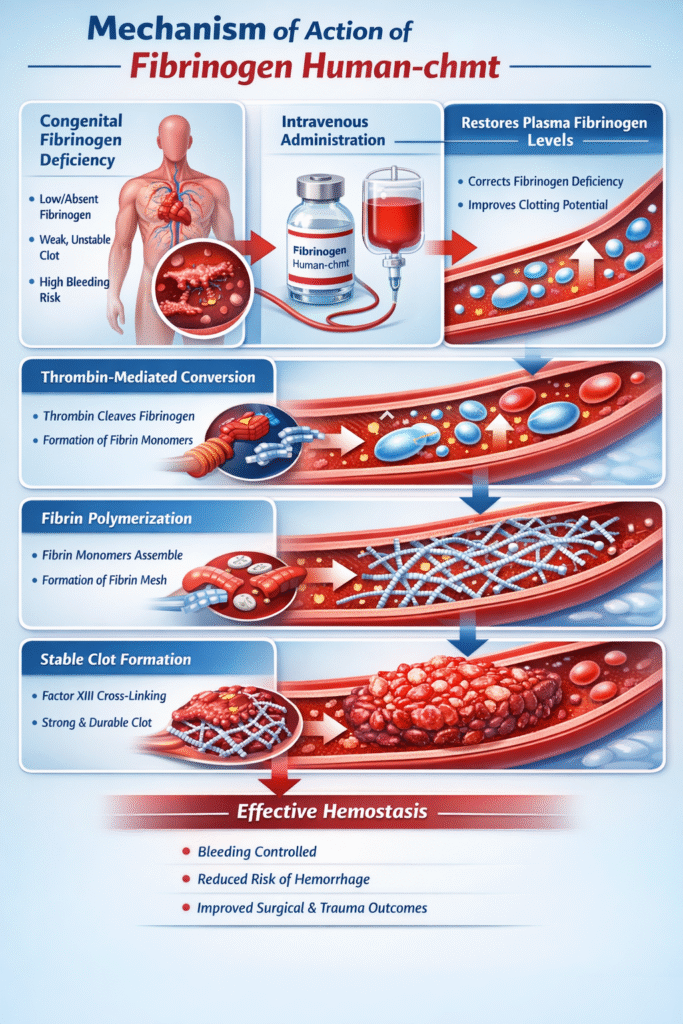
Mechanism of Action (MOA)
The mechanism of action of fesilty is directly aligned with the physiological role of fibrinogen in the coagulation cascade.
Detailed Mechanistic Pathway
- Intravenous Administration and Plasma Distribution
After infusion, fibrinogen human-chmt rapidly distributes within the intravascular space, increasing circulating fibrinogen concentrations. - Thrombin-Mediated Cleavage
During coagulation, thrombin enzymatically cleaves fibrinogen molecules into fibrin monomers. - Fibrin Polymerization
These monomers spontaneously assemble into long fibrin polymers that interlace to form a fibrin network. - Clot Stabilization and Strengthening
Activated factor XIII cross-links the fibrin strands, resulting in a stable, elastic clot capable of withstanding blood flow and mechanical stress.
By restoring this final common pathway of coagulation, fibrinogen human-chmt effectively halts bleeding and supports tissue repair.
Pharmacokinetics and Pharmacodynamics
Fibrinogen human-chmt displays predictable pharmacokinetic behavior, which is crucial for individualized dosing.
- Route: Intravenous
- Bioavailability: Complete (100%)
- Plasma Half-life: Approximately 3 to 5 days
- Distribution: Primarily intravascular
- Elimination: Normal fibrin metabolism and clearance pathways
Pharmacodynamic response is measured by increases in plasma fibrinogen levels and improved clot firmness.
Clinical Uses
Fesilty is clinically used in several high-risk bleeding scenarios, including:
- Acute bleeding in congenital fibrinogen deficiency
- Surgical and perioperative bleeding management
- Trauma-associated hypofibrinogenemia
- Prevention of bleeding during invasive procedures
In emergency situations, fibrinogen human-chmt allows rapid correction of fibrinogen levels without the large fluid volumes associated with plasma transfusions.
Dosage Principles and Administration
Dosing of fesilty is clinically used in several high-risk bleeding scenarios, including:
is individualized and based on:
- Patient body weight
- Baseline fibrinogen concentration
- Desired target fibrinogen level
- Clinical severity of bleeding
The product is reconstituted under aseptic conditions and infused intravenously at a controlled rate. Laboratory monitoring guides repeat dosing.
Side Effects and Safety Profile
Common Side Effects
- Headache
- Fever
- Nausea
- Pain in extremities
- Mild infusion-site reactions
These effects are usually transient and resolve without intervention.
Serious Adverse Events
Rare but serious adverse events associated with fibrinogen human-chmt include:
- Thromboembolic complications
- Hypersensitivity or allergic reactions
- Potential immune responses
Careful patient selection and monitoring reduce these risks.
Thrombosis Risk and Monitoring
Because fibrinogen promotes clot formation, excessive levels may increase the risk of thrombosis. Patients receiving fibrinogen human-chmt should be monitored for:
- Plasma fibrinogen concentration
- Signs of venous or arterial thrombosis
- Coagulation parameters when clinically indicated
Balancing hemostasis with thrombosis risk is a key aspect of therapy.
Drug Interactions of Fibrinogen Human-chmt
Although fibrinogen human-chmt does not undergo hepatic metabolism, interactions may occur through opposing effects on coagulation.
Relevant Drug Classes
- Anticoagulants: May reduce therapeutic effectiveness
- Antiplatelet agents: May impair clot stability
- Other coagulation factor concentrates: Increased clotting risk if overdosed
Clinical judgment is essential when combining these therapies.
Use in Special Populations
Pediatric Patients
Approved for use with weight-based dosing and careful monitoring.
Pregnancy
May be considered when benefits outweigh potential risks in severe bleeding.
Elderly Patients
Require cautious dosing due to higher baseline thrombotic risk.
Comparison With Traditional Blood Products
Compared with cryoprecipitate or fresh frozen plasma, fibrinogen human-chmt offers:
- Standardized fibrinogen content
- Faster preparation and administration
- Lower infusion volume
- Reduced transfusion-related risks
These advantages support its growing use in clinical practice.
Future Role of Fibrinogen Human-chmt
As patient blood management strategies evolve, fibrinogen human-chmt is expected to play an expanding role in trauma care, major surgery, and obstetric hemorrhage. Ongoing clinical experience continues to refine its optimal use.
Conclusion
Fibrinogen human-chmt, approved on December 16, 2025, is a modern fibrinogen replacement therapy with a well-defined mechanism of action, predictable pharmacokinetics, and a favorable safety profile. By addressing the central role of fibrinogen in coagulation, it provides effective and targeted treatment for acute bleeding in congenital fibrinogen deficiency and beyond.

