Table of Contents
Introduction
The Elamipretide FDA Approval is a revolutionary step in the treatment of one of the world’s rarest and most devastating mitochondrial diseases – Barth syndrome. After years of research, advocacy and clinical trials, the US Food and Drug Administration has granted accelerated approval to Forzinity (elamipretide) injection, making it the first approved therapy for patients with this life-threatening disease.
In this blog post, we’ll explore what elamipretide is, how it works, the details of its FDA approval, and what this decision means for patients, families and the future of rare disease treatment.
What Is Elamipretide?
Elamipretide, also known by the brand name Forzinity, is a first-in-class therapeutic peptide designed to restore mitochondrial function. Mitochondria are the powerhouses of cells, responsible for producing the energy our bodies need to survive. When mitochondria are damaged, cells struggle to produce energy efficiently, leading to a number of serious health problems.
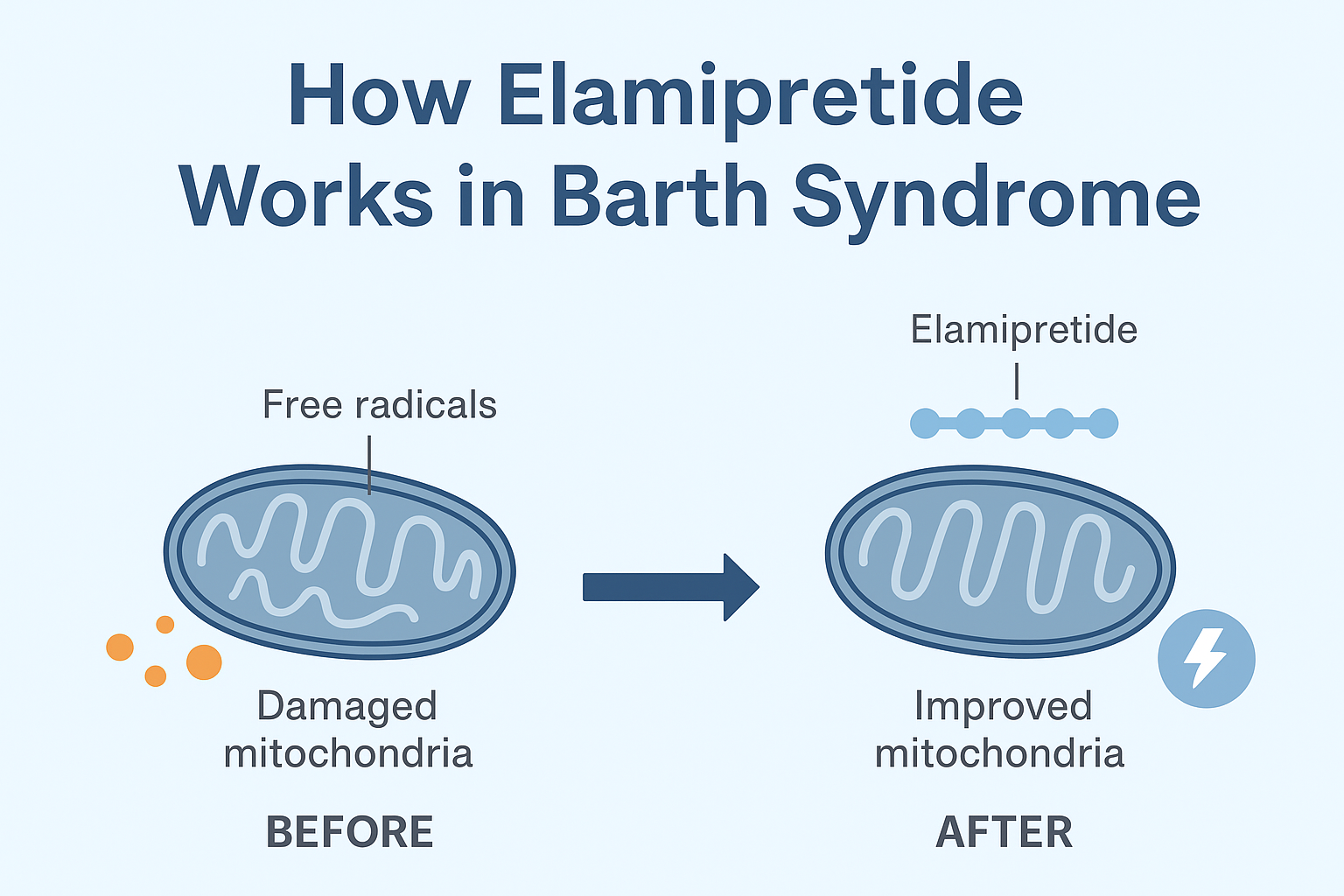
Elamipretide works by targeting cardiolipin, an important phospholipid found in the inner mitochondrial membrane. In patients with mitochondrial disorders such as Barth syndrome, cardiolipin becomes abnormal or unstable, which disrupts mitochondrial energy production. Elamipretide binds to cardiolipin, stabilizing it and restoring normal mitochondrial function. This helps improve energy metabolism, muscle strength and overall cell function.
This medication is given by subcutaneous injection and is designed for patients weighing at least 30 kilograms.
Understanding Barth Syndrome
To truly understand the significance of the FDA approval of elamipretide, it is important to understand the condition it treats – Barth syndrome.
Barth syndrome is an extremely rare genetic condition that primarily affects males. It is caused by mutations in the TAZ gene, which interfere with the body’s ability to produce normal cardiolipin – an essential component of healthy mitochondria. Without functional cardiolipin, cells cannot efficiently produce energy, especially in the heart and skeletal muscles.
Key Symptoms of Barth Syndrome
- Dilated cardiomyopathy (weak and enlarged heart)
- Heart failure
- Skeletal muscle weakness and fatigue
- Growth delay and short stature
- Neutropenia (low white blood cell count leading to frequent infections)
- Exercise intolerance and low stamina
The disease can be life-threatening, particularly in early childhood. Many patients face significant health challenges throughout their lives, with limited treatment options available — until now.
The Science Behind Elamipretide
The approval of elamipretide represents more than just another drug approval; it’s a milestone in mitochondrial medicine.
Mechanism of Action
• Elamipretide is a mitochondrial-targeting peptide that crosses cell membranes and specifically binds to cardiolipin, which is important for maintaining mitochondrial membrane integrity and function.
• By binding to cardiolipin, elamipretide stabilizes mitochondrial structure, reduces oxidative stress, and increases the efficiency of energy production (ATP synthesis).
• The result is improved muscle energy balance, better muscle strength, and improved cardiac function – all important areas affected in Barth syndrome.
In clinical and experimental studies, elamipretide has shown the ability to improve exercise tolerance, increase muscle strength, and support cardiac function, offering hope to patients who previously had no approved therapeutic options.
Clinical Trials Supporting Elamipretide
The journey toward elamipretide FDA approval has been backed by years of clinical studies.
1. The TAZPOWER Study
The pivotal trial, known as TAZPOWER, evaluated elamipretide in patients with Barth syndrome. It was a double-blind, placebo-controlled study followed by an open-label extension.
Key findings included:
- Noticeable improvements in muscle strength and endurance.
- Enhanced exercise capacity measured through walking tests.
- Improvement in quality of life scores and cardiac function in many participants.
2. Natural History Study
To further support its efficacy, researchers conducted a long-term natural history study comparing the progression of treated versus untreated patients. This data helped confirm that elamipretide offers real, measurable clinical benefits in a disease that typically worsens over time.
3. Safety and Tolerability
Elamipretide has demonstrated a favorable safety profile. The most common side effects reported were mild injection site reactions such as redness or swelling. Overall, the therapy was well tolerated, with no serious drug-related adverse events observed in the majority of participants.
Elamipretide FDA Approval: A Historic Milestone
In September 2025, the U.S. Food and Drug Administration granted accelerated approval to Forzinity (elamipretide) as the first treatment for Barth syndrome in patients weighing at least 30 kg.
The Elamipretide FDA Approval represents a monumental achievement in rare disease medicine and highlights the FDA’s commitment to supporting therapies for conditions that have no existing treatment options.
Key Highlights of the Elamipretide FDA Approval
- Approval Date: September 25, 2025
- Brand Name: Forzinity
- Generic Name: Elamipretide
- Manufacturer: Stealth BioTherapeutics
- Indication: Treatment of Barth syndrome in patients ≥ 30 kg
- Regulatory Pathway: Accelerated approval based on improvement in knee extensor muscle strength, a surrogate endpoint reasonably likely to predict clinical benefit.
Why the Accelerated Approval Matters
Drugs that treat serious or life-threatening conditions and that demonstrate significant potential based on surrogate endpoints are granted expedited approval. For Barth syndrome, where large-scale clinical trials are impractical due to the extremely small number of patients, this pathway provides patients with early access to life-saving treatments while additional studies confirm long-term benefits. The Elamipretide FDA Approval through this mechanism highlights the FDA’s flexibility and patient-centered approach to addressing urgent unmet medical needs.
What This Means for Patients and Families
The elamipretide FDA approval is life-changing news for individuals and families affected by Barth syndrome.
1. First-Ever Approved Treatment
For decades, patients relied solely on supportive therapies such as heart medications, antibiotics, and nutritional management. Elamipretide now provides the first targeted therapy that directly addresses the root cause — mitochondrial dysfunction.
2. Improved Quality of Life
By enhancing energy production, elamipretide has the potential to reduce fatigue, improve exercise tolerance, and strengthen heart function, allowing patients to engage in daily activities with greater ease and independence.
3. Hope for the Future
Families affected by Barth syndrome have long advocated for medical recognition and research. The Elamipretide FDA Approval not only validates their perseverance but also provides hope that future therapies will continue to emerge for other mitochondrial and genetic diseases.
Ongoing Commitments and Post-Marketing Studies
Since the approval is based on accelerated approval, the manufacturer is required to conduct post-marketing confirmatory trials. These studies will further evaluate whether the observed improvements in muscle strength translate to meaningful clinical benefits in daily life, such as walking ability and cardiac performance.
If confirmatory results are positive, elamipretide’s approval may be converted to full FDA approval. However, if the data fail to confirm clinical benefit, the agency may reconsider the authorization. This process ensures that patients continue to receive safe and effective therapy supported by long-term data.
Challenges Ahead
While the Elamipretide FDA Approval is an exciting milestone, it also comes with challenges:
- Limited Eligibility – Currently, only patients weighing 30 kg or more are eligible. Younger children and infants remain excluded until further studies expand its indication.
- Access and Cost – As a treatment for an ultra-rare disease, accessibility and pricing will be key issues for healthcare systems and families.
- Monitoring Safety and Efficacy – Continued safety surveillance and clinical monitoring are essential to ensure the therapy’s benefits outweigh risks over time.
Despite these challenges, the path forward is filled with optimism and progress for the Barth syndrome community.
How Elamipretide Is Changing Rare Disease Treatment
The success of elamipretide paves the way for future innovations in mitochondrial medicine. The Elamipretide FDA Approval demonstrates how targeted therapies can emerge from cutting-edge molecular science, even in the smallest patient populations.
It also sets a regulatory precedent — proving that meaningful treatments for ultra-rare conditions are possible through collaboration between researchers, patient organizations, and regulatory agencies.
Elamipretide’s mechanism of restoring mitochondrial function could have implications for other disorders involving mitochondrial dysfunction, including heart failure, muscular dystrophy, and neurodegenerative diseases. Continued research will reveal how broad its therapeutic potential may be.
Conclusion
The Elamipretide FDA Approval to treat rare diseases is a milestone, offering new hope to patients with Barth syndrome who previously had no effective options. It reflects the power of modern biotechnology and compassionate regulation – where science, perseverance, and advocacy come together to change lives.
While more research is underway, Elamipretide FDA Approval represents a beacon of progress and a glimpse of a future where real, life-changing treatments may be available for even rare diseases.

1 thought on “Elamipretide FDA Approval: A Breakthrough Treatment for Barth Syndrome”