Table of Contents
Introduction
On August 6, 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval to dordaviprone (brand name: Modeyso), making history as the first systemic therapy for patients with diffuse midline glioma (DMG) harboring the H3 K27M mutation. This approval is a landmark moment, offering hope for children and adults battling this devastating and aggressive brain tumor.
For decades, treatment options for diffuse midline glioma were extremely limited, with radiation being the only standard approach. The dordaviprone FDA approval introduces a new pathway of care, changing how physicians and patients can approach this condition.
This blog will cover everything you need to know about dordaviprone, including:
- What diffuse midline glioma is
- Why the H3 K27M mutation matters
- How dordaviprone works
- The FDA approval details
- Clinical trial evidence
- Side effects and safety information
- Future directions for research and therapy.
What Is Diffuse Midline Glioma (DMG)?
Diffuse midline glioma (DMG) is a rare and aggressive brain tumor that primarily affects children but can also occur in adults. DMGs usually arise in midline structures of the brain such as the:
- Thalamus
- Brainstem (pons)
- Spinal cord
Key Characteristics of DMG:
- Rapid progression and resistance to therapy
- Poor prognosis (median survival less than 12–15 months)
- Commonly linked with the H3 K27M gene mutation
- Symptoms include headaches, balance problems, weakness, vision issues, and difficulty with movement
For years, there were no effective systemic therapies for DMG. Standard radiation offered temporary relief but failed to significantly extend survival. This is why the dordaviprone FDA approval is considered a milestone achievement in neuro-oncology
The Role of the H3 K27M Mutation
The H3 K27M mutation occurs in histone proteins and alters the epigenetic regulation of gene expression in tumor cells. This leads to:
- Uncontrolled tumor growth
- Resistance to many standard cancer therapies
- Aggressive clinical behavior
Targeting tumors with this specific mutation has been a major focus of cancer research in recent years. Dordaviprone is the first FDA-approved therapy designed for patients with progressive DMG carrying this mutation.
Dordaviprone: What You Need to Know
Generic & Brand Name
- Generic Name: Dordaviprone
- Brand Name: Modeyso
Indication
Approved for adult and pediatric patients with progressive DMG harboring H3 K27M mutation who have already received prior therapy.
Approval Pathway
The FDA granted accelerated approval based on clinical trial evidence showing tumor response rates and durability of response in patients with limited treatment options.
This means further confirmatory studies are required, but the therapy is already available for eligible patients.
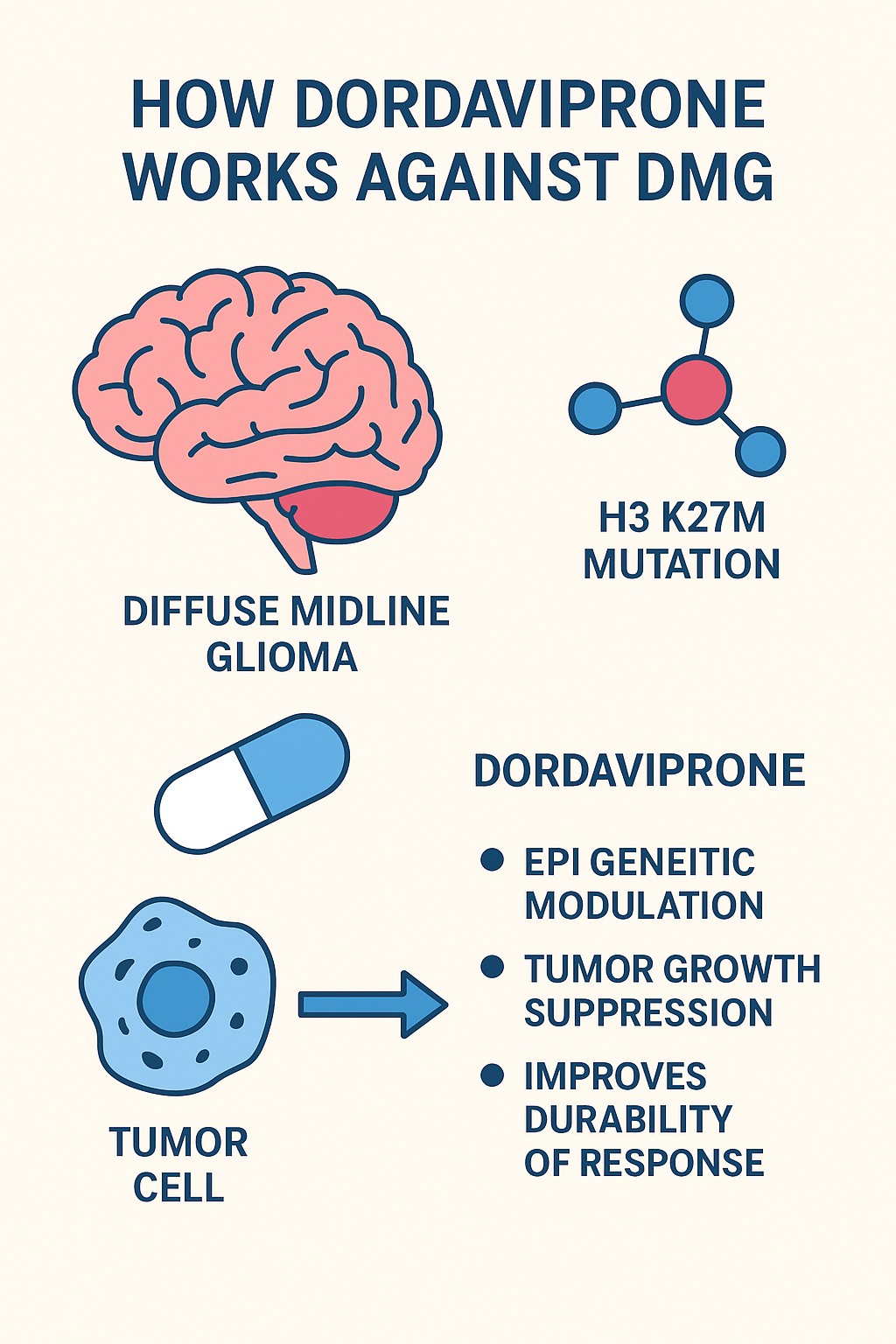
How Dordaviprone Works
Dordaviprone is a targeted oral therapy that interferes with the epigenetic mechanisms driving tumor growth in DMG.
Mechanism of Action (MoA):
- Targets H3 K27M Mutation: Specifically acts on cells harboring this mutation.
- Epigenetic Modulation: Restores normal gene regulation by reversing abnormal histone methylation patterns.
- Tumor Growth Suppression: Slows down proliferation of cancerous cells.
- Improves Durability of Response: Provides more sustained disease control compared to radiation alone.
By focusing on the genetic driver of DMG, dordaviprone offers a precision medicine approach to treatment.
Clinical Trial Evidence Supporting Dordaviprone FDA Approval
The FDA approval of dordaviprone was based on results from pivotal clinical studies in patients with progressive DMG.
Key Findings:
- Objective Response Rate (ORR): A significant proportion of patients achieved measurable tumor shrinkage.
- Durability of Response: Responses were sustained for months, showing the drug’s long-term benefit.
- Pediatric and Adult Efficacy: Both groups demonstrated meaningful improvements.
- Manageable Safety Profile: Most side effects were tolerable and reversible with dose adjustments.
These results provided enough evidence for the FDA to grant accelerated approval, offering urgent access while further studies continue.
Benefits of Dordaviprone for Patients
The dordaviprone FDA approval has several major benefits:
- First-Ever Systemic Therapy for DMG – Patients now have a drug therapy beyond radiation.
- Hope for Pediatric Patients – Children with DMG now have an FDA-approved treatment option.
- Targeted Precision Therapy – Focuses on the genetic mutation driving the cancer.
- Improved Survival Outlook – Early evidence suggests potential for extending life expectancy.
- Oral Administration – Easier to manage compared to invasive procedures.
Side Effects of Dordaviprone
Like all cancer drugs, dordaviprone has potential side effects. Most are mild to moderate, but some require medical monitoring.
Common Side Effects:
- Fatigue
- Nausea and vomiting
- Headache
- Loss of appetite
- Low blood counts (anemia, neutropenia)
Serious Side Effects (Less Common):
- Liver enzyme elevation
- Neurological effects
- Severe cytopenias
Doctors will conduct regular blood tests and imaging scans to monitor safety and effectiveness.
Safety Considerations
- Pregnancy & Breastfeeding: Not recommended due to potential risks to fetus or infant.
- Drug Interactions: May interact with other cancer drugs or supportive medications.
- Monitoring Required: Patients should undergo MRI scans and lab tests to track response and toxicity.
Dordaviprone FDA Approval: Why It Matters
The dordaviprone FDA approval marks a historic moment in the fight against pediatric and adult brain cancers. Until now, families faced a future with almost no systemic options for DMG.
This approval:
- Validates years of research on epigenetic-targeted therapies.
- Opens the door for future drug development in rare brain cancers.
- Provides immediate access to a therapy for patients with progressive, life-threatening disease.
Future Research and Outlook
The accelerated approval means that confirmatory studies are still ongoing. Future areas of research include:
- Combination Therapies: Using dordaviprone with radiation or immunotherapy.
- Earlier-Line Treatment: Studying its effect if given at diagnosis, not just after progression.
- Expanded Indications: Potential testing in other gliomas or cancers with histone mutations.
The medical community is hopeful that dordaviprone could become the foundation for broader treatment strategies in neuro-oncology.
Conclusion
The dordaviprone FDA approval represents a turning point in brain cancer treatment, especially for children and adults with diffuse midline glioma (DMG). For the first time, patients have a systemic, mutation-targeted therapy that offers real hope in the face of an otherwise grim diagnosis.
While challenges remain and more studies are needed, the approval of dordaviprone (Modeyso) is a historic step toward precision oncology in neuro-oncology. It gives patients, caregivers, and clinicians a reason to believe in a brighter future.

