Table of Contents
Introduction
Hereditary angioedema (HAE) is a rare genetic disorder that causes recurrent swelling of various tissues, such as the face, limbs, abdomen, or airways. These attacks can be painful, disabling, and sometimes life-threatening (especially if the airways are involved). While conventional treatments have made great progress, many patients still face uncertainty and recurrent episodes.
Enter donidalorsen, a ligand-conjugated antisense oligonucleotide therapy designed to prevent HAE attacks by targeting an underlying molecular pathway. What was once investigational has now reached a major milestone: US FDA approval on August 21, 2025, for the prevention of HAE in patients 12 years of age and older.
In this updated blog post, we explore what donidalorsen is, how it works, the evidence supporting its use, its safety profile, comparisons to existing treatments, and what the future holds for HAE patients.
What Exactly Is Donidalorsen?
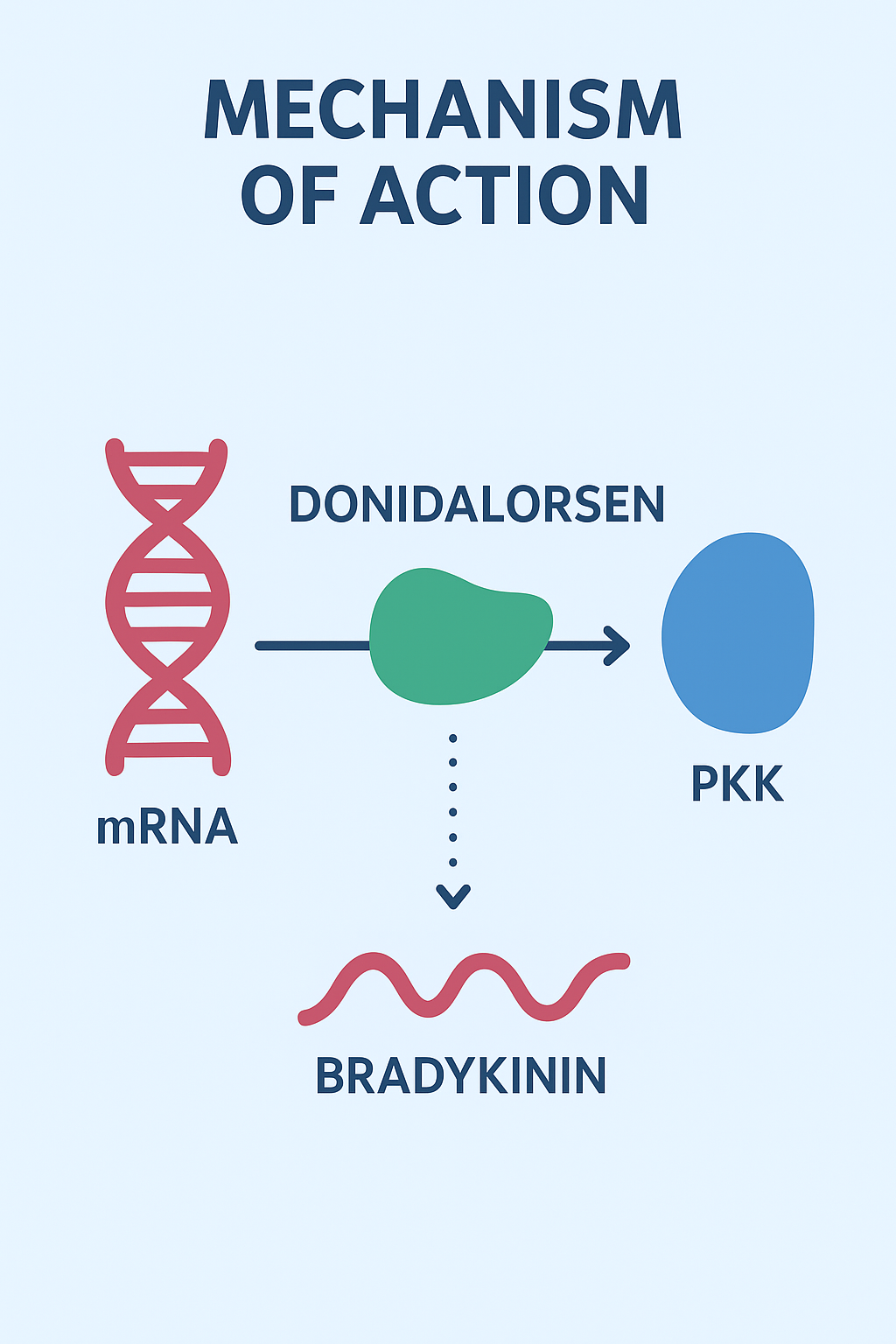
Donidalorsan (brand name Donzera) is a prekallikrein-directed antisense oligonucleotide therapy. It works by binding to the messenger RNA (mRNA) of the KLKB1 gene (which encodes prekallikrein or PKK), causing degradation of that mRNA and thereby reducing the production of the PKK protein.
In effect, donidalorsan reduces the production of bradykinin (a potent mediator of vascular permeability and inflammation) by reducing the upstream supply of prekallikrein. This mechanistic targeting places it in a new class of RNA-targeted therapies – drugs that target the level of RNA rather than proteins or receptors.
Because it is ligand-conjugated, the molecule is designed to be efficiently delivered to specific cells so that it can precisely target its target.
FDA Approval & Regulatory Milestone
An important update is that donidalorsen is no longer just for investigational use – it has now been approved by the US Food and Drug Administration as a preventative for HAE attacks in individuals 12 years of age and older, effective August 21, 2025.
The approval was based on data from the Phase 3 OASIS-HAE trial, which showed that donidalorsen reduced the frequency of monthly HAE attacks by 81% compared to placebo over 24 weeks when dosed every 4 weeks.
Under its trade name, Donzera, donidalorsen becomes the first RNA-targeted therapy approved for HAE prevention.
Mechanism of Action
To appreciate its potential, it helps to understand precisely how Donidalorsen interacts with the disease pathway:
- mRNA targeting — Donidalorsen binds specifically to mRNA transcripts of the KLKB1 gene (which codes for prekallikrein).
- mRNA degradation — This binding triggers RNase H-mediated cleavage of the mRNA, thereby reducing translation into the PKK protein.
- Lower PKK levels — With reduced PKK available, the cascade that converts high molecular weight kininogen to bradykinin is curtailed.
- Reduced bradykinin — Less bradykinin means less vascular leakage and fewer swelling episodes.
Because it works upstream, it offers a prophylactic (preventive) effect rather than treating attacks after they begin.
Clinical Evidence & Trial Data
Phase 3: OASIS-HAE
The pivotal trial driving FDA approval was OASIS-HAE, a global, randomized, double-blind, placebo-controlled study. Key findings:
- Donidalorsen, dosed once every 4 weeks, achieved an 81% reduction in monthly HAE attacks compared to placebo over a 24-week period.
- The safety profile was acceptable, with no unexpected serious adverse events leading to discontinuation.
- Secondary endpoints included reductions in rescue medication usage, improvement in quality-of-life scores, and fewer moderate-to-severe attacks.
Earlier Phases & Extensions
- Phase 2 and open-label extension studies showed consistent reductions in attack rates with good tolerability.
- These studies laid the foundation for the robust design and expectations of the Phase 3 trial.
- In the longer-term extensions, Donidalorsen continued to demonstrate durability in effect.
Overall, the clinical program has moved from proof-of-concept to confirmatory evidence that supports its efficacy in real-world settings.
Approved Indication & Usage
With FDA approval, Donidalorsen (Dawnzera) is now indicated for prophylaxis to prevent attacks of hereditary angioedema in adult and pediatric patients aged 12 years and older.
Key points about its usage:
- It is delivered via subcutaneous injection.
- In the pivotal trial, the once-monthly (Q4W) regimen was used. Extended intervals (e.g. every 8 weeks) are being explored depending on patient response.
- Patients and clinicians must monitor for hypersensitivity or injection-related reactions.
It is anticipated that Donidalorsen will be available for prescribing in the U.S. following its commercial launch.
Potential Benefits & Clinical Advantages
- Substantial Reduction in Attack Rate – 81% fewer attacks compared to placebo.
- Preventive Mode – Designed to prevent episodes rather than treat them after onset.
- Less Frequent Dosing – Potential for longer dosing intervals.
- Improved Quality of Life – Fewer disruptions to work, school, travel, and family life.
- First RNA-Targeted Option – A new milestone for HAE prophylaxis.
- Reduced Emergency Visits – Preventing airway involvement could lower hospital admissions.
Safety Profile & Possible Risks
In both clinical trials and extension studies, the safety signals of Donidalorsen have been acceptable, though monitoring remains essential. Some noted risks include:
- Injection site reactions
- Upper respiratory infections
- Urinary tract infections
- Gastrointestinal discomfort
- Hypersensitivity or allergic reactions (rare but possible)
These side effects were generally mild to moderate and manageable. Because Donidalorsen acts at the RNA level, long-term surveillance is needed to confirm safety across years of use.
Comparison with Existing HAE Therapies
| Class | Example Drugs | Mode of Use | Pros | Cons |
| On-demand (acute) | Icatibant, C1-INH concentrate | Used during attacks | Rapid relief | No preventive effect |
| Prophylactic biologics | Lanadelumab, Berotralstat | Regular dosing | Effective prevention | Frequent dosing burden |
| Oral option | Berotralstat | Daily oral capsule | Convenient oral use | Requires daily adherence |
| RNA therapy (new) | Donidalorsen (Dawnzera) | Monthly or less frequent injection | Upstream suppression, durable effect | Long-term safety monitoring needed |
Donidalorsen’s unique RNA-targeted mechanism positions it as a longer-lasting, more preventive therapy compared to existing approaches.
Real-World Considerations & Challenges
- Cost and access – As a novel therapy, pricing and insurance coverage will play a major role.
- Long-term monitoring – RNA therapies require careful pharmacovigilance.
- Patient selection – Ideal for patients with frequent or severe attacks.
- Global rollout – Regulatory approvals in other countries are still pending.
- Self-injection training – Patients may need support to administer doses correctly.
Patient Perspective
For patients, living with HAE often means constant fear of unpredictable attacks. Donidalorsen offers new hope: fewer attacks, more stability, and reduced anxiety about airway obstruction. This can translate into improved mental well-being and the ability to live a more normal, independent life.
Expert Outlook & Future Directions
Experts believe Donidalorsen’s approval represents a new era for RNA-based therapies in rare diseases. Future research may explore:
- Longer dosing intervals (every 8 weeks or beyond).
- Use in younger children.
- Real-world effectiveness studies.
- Personalized treatment strategies based on biomarkers.
Conclusion
With FDA approval in August 2025, donidalorsen (Donzera) became the first RNA-targeted prophylactic treatment for hereditary angioedema. Its ability to reduce attack frequency, provide long-term stability, and improve patients’ quality of life is a significant milestone in HAE care. While challenges such as accessibility, cost, and long-term monitoring remain, donidalorsen offers new hope and represents a major advance in the treatment of rare diseases. For many patients, this could mean fewer hospital visits, less anxiety, and a more predictable future.

