Table of Contents
Introduction:
The landscape of therapeutic options for managing bone-related complications in cancer and other skeletal disorders has been significantly advanced by the introduction of biologic agents. Among these, denosumab, a human monoclonal antibody, has established itself as a cornerstone treatment. The emergence of biosimilars, which are highly similar to an already approved biological medicine (the reference product), represents a crucial step in expanding patient access and reducing healthcare costs.
Denosumab-mobz is one such biosimilar, offering an alternative to the reference product for patients requiring this vital therapy. This development is not merely a matter of pharmaceutical competition; it is a public health victory, promising to make life-changing treatments more accessible to a broader population suffering from debilitating bone conditions. The complexity of manufacturing a biologic like denosumab means that the development of a biosimilar like Denosumab-mobz is a testament to advanced biotechnological capabilities.
This comprehensive guide, written by a senior blog writer with a focus on technical accuracy and SEO optimization, delves into the critical aspects of Denosumab-mobz. We will explore its intricate mechanism of action (MOA), detail its FDA approval and approved indications, provide an in-depth look at its potential side effects, and clarify its drug interaction profile.
Understanding Denosumab-mobz and its FDA Journey
Denosumab-mobz is the non-proprietary name assigned to a specific biosimilar version of denosumab. The suffix “-mobz” is a unique identifier, or a four-letter non-proprietary suffix, designated by the FDA to distinguish this particular biosimilar from the reference product and other potential biosimilars. This naming convention is part of the FDA’s effort to ensure pharmacovigilance and clear communication regarding biological products.
The specific product, marketed under the trade name Oziltus, has demonstrated high similarity to the original denosumab product, Xgeva, in terms of safety, purity, and potency . This demonstration of biosimilarity is a rigorous process, involving extensive analytical, non-clinical, and clinical data to prove that there are no clinically meaningful differences between the biosimilar and the reference product.
The FDA approval of Denosumab-mobz is a landmark event, confirming that it is biosimilar to the reference product and can be used for the same approved indications. The initial approval of the reference product, denosumab, dates back to 2010. The subsequent approval of Denosumab-mobz (Oziltus) in late 2025, with a marketing start date of December 23, 2025, signifies a major advancement in the availability of this treatment . This biosimilar pathway ensures that patients can receive a high-quality, effective treatment option, which is particularly important given the chronic nature of the conditions it treats.
The introduction of Denosumab-mobz is expected to foster competition and potentially lower the financial burden associated with this class of drugs, thereby increasing patient access globally. The rigorous regulatory process for Denosumab-mobz provides a high level of confidence in its therapeutic equivalence.
The Significance of Biosimilarity
Biosimilars are distinct from generic drugs. While generics are chemically identical to their reference products, biosimilars are complex biological molecules derived from living systems, making an exact replication impossible. Instead, the biosimilar manufacturer must prove that the product is “highly similar” to the reference product and has “no clinically meaningful differences.” This includes comparing the structure, function, animal toxicity, human pharmacokinetics, pharmacodynamics, immunogenicity, and clinical efficacy and safety.
For Denosumab-mobz, this means that patients and prescribers can expect the same clinical outcomes as with the reference product. This is a crucial point for the acceptance and adoption of Denosumab-mobz in clinical practice.
FDA-Approved Indications
The indications for Denosumab-mobz mirror those of its reference product, primarily focusing on conditions characterized by excessive bone breakdown (resorption). These include:
- Prevention of Skeletal-Related Events (SREs): This is a primary indication for patients with multiple myeloma and those with bone metastases from solid tumors. SREs include pathological fractures, spinal cord compression, and the need for radiation or surgery to bone. The use of Denosumab-mobz in this setting is vital for improving quality of life and reducing morbidity.
- Treatment of Giant Cell Tumor of Bone (GCTB): It is indicated for adults and skeletally mature adolescents with GCTB that is unresectable or where surgical resection is likely to result in severe morbidity. This provides a non-surgical option for a challenging bone tumor.
- Treatment of Hypercalcemia of Malignancy (HCM): It is used for the treatment of high blood calcium levels caused by cancer that is refractory to bisphosphonate therapy. This is often a life-threatening condition, and the rapid action of Denosumab-mobz is highly beneficial.
The Mechanism of Action (MOA)
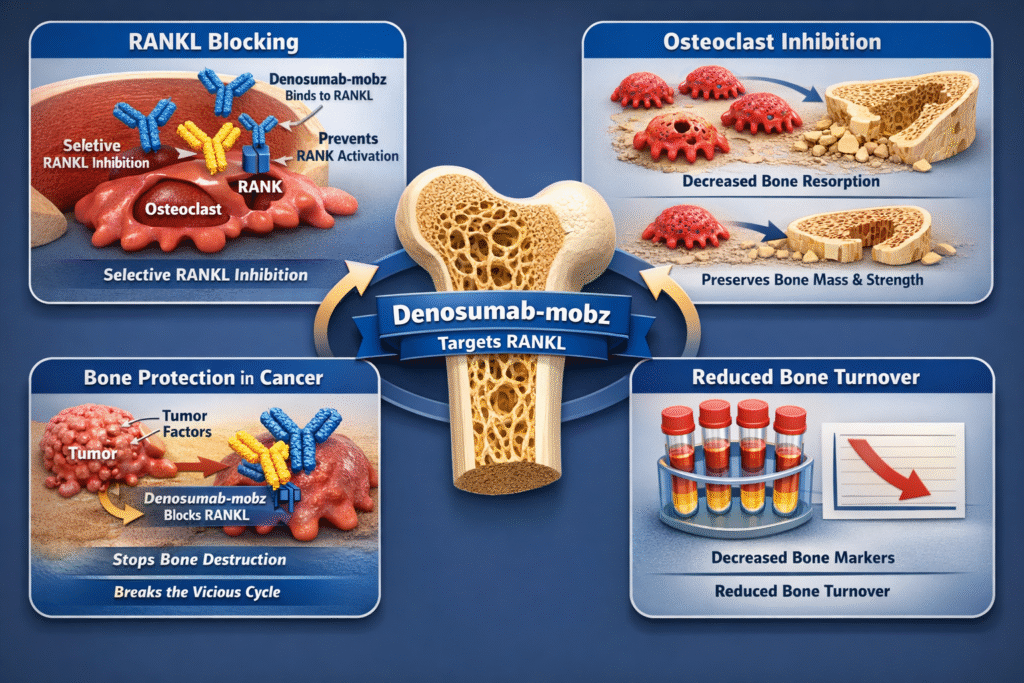
The therapeutic efficacy of Denosumab-mobz stems from its highly specific and targeted mechanism of action, which directly intervenes in the bone remodeling cycle. Bone is a dynamic tissue constantly undergoing a process of breakdown (resorption) by cells called osteoclasts and formation by cells called osteoblasts. In healthy individuals, these processes are tightly coupled and balanced. However, in many diseases, particularly cancer-related bone disease, this balance is tipped towards excessive resorption, leading to bone destruction, pain, and complications. The precise intervention offered by Denosumab-mobz is what makes it a powerful therapeutic agent.
The Bone Remodeling Cycle and Imbalance
The continuous process of bone remodeling is essential for maintaining skeletal integrity and mineral homeostasis. Osteoclasts are large, multinucleated cells that dissolve bone mineral and matrix, a process known as bone resorption. Osteoblasts are responsible for forming new bone tissue. The activity of these two cell types is regulated by a complex signaling system, the most critical component of which is the RANKL-RANK-OPG pathway. In pathological states, such as the presence of bone metastases, tumor cells often secrete factors that dramatically increase the expression of RANKL, leading to an overabundance of active osteoclasts and subsequent excessive bone destruction.
The RANKL-RANK Pathway
Denosumab-mobz is a human IgG2 monoclonal antibody that targets the Receptor Activator of Nuclear factor Kappa-B Ligand (RANKL). This protein is a key mediator in the communication between osteoblasts and osteoclasts.
- RANKL: Expressed on the surface of osteoblasts and other cells, RANKL acts as a signaling molecule.
- RANK: The receptor for RANKL, found on the surface of osteoclast precursors and mature osteoclasts.
When RANKL binds to RANK, it triggers a cascade of events that promote the formation, function, and survival of osteoclasts. This leads to increased bone resorption. The molecular structure of Denosumab-mobz is specifically engineered to mimic the natural inhibitor of RANKL, osteoprotegerin (OPG), but with a much higher affinity and stability.
How Denosumab-mobz Works at the Molecular Level
Denosumab-mobz acts as a decoy for RANKL. By binding to soluble and transmembrane RANKL with high affinity and specificity, it prevents RANKL from activating its receptor, RANK, on the osteoclast surface . This inhibition effectively shuts down the signaling pathway that drives osteoclast activity. The binding of Denosumab-mobz to RANKL is highly selective, ensuring that other critical signaling pathways are not inadvertently affected. This targeted approach minimizes off-target effects, a significant advantage of monoclonal antibody therapies.
The consequence of this action is a rapid and profound decrease in the number and function of osteoclasts. This leads to a significant reduction in bone resorption, thereby preserving bone mass and strength. In the context of cancer, where tumors often secrete factors that increase RANKL production, the action of Denosumab-mobz is critical in preventing the vicious cycle of bone destruction and tumor growth. The precision of Denosumab-mobz in targeting this single pathway is what makes it such an effective agent in skeletal protection. The clinical effect is a measurable decrease in bone turnover markers, confirming the potent anti-resorptive activity of Denosumab-mobz.
Potential Side Effects and Safety Profile
While Denosumab-mobz is a highly effective treatment, it is associated with a number of important and potentially serious adverse reactions. A thorough understanding of the safety profile is paramount for patients and healthcare providers managing treatment with Denosumab-mobz. The biosimilar nature of the drug means its safety profile is expected to be identical to the reference product, but vigilance remains key.
Serious Warnings and Precautions
The FDA label for Denosumab-mobz includes several serious warnings, which highlight the need for careful patient monitoring:
1. Hypocalcemia (Low Blood Calcium)
This is a significant risk, especially in the first weeks of therapy. Denosumab-mobz can cause severe, symptomatic hypocalcemia, which can be fatal. Pre-existing hypocalcemia must be corrected before initiating therapy. Patients must be adequately supplemented with calcium and vitamin D throughout treatment. Monitoring of calcium levels is essential, particularly in patients with renal impairment, as they are at higher risk .
The mechanism behind this is the rapid suppression of bone resorption, which reduces the release of calcium from bone into the bloodstream. This effect is particularly pronounced in patients with compromised kidney function, who may have difficulty regulating calcium and phosphate levels. Healthcare providers must educate patients on the symptoms of hypocalcemia, such as muscle cramps, spasms, or numbness and tingling in the fingers, toes, or around the mouth.
2. Osteonecrosis of the Jaw (ONJ)
ONJ is a rare but serious adverse reaction characterized by exposed bone in the jaw that fails to heal. The risk of ONJ is higher in patients with cancer, those receiving chemotherapy, corticosteroids, or those with poor oral hygiene. Patients should maintain good oral hygiene, and invasive dental procedures should be avoided during treatment with Denosumab-mobz.
A dental examination with appropriate preventive dentistry should be performed prior to initiation of Denosumab-mobz therapy. For patients who develop ONJ, treatment may involve conservative management, antibiotics, and in some cases, surgical debridement, though surgery should be approached with caution. The risk of ONJ is a major consideration when prescribing Denosumab-mobz.
3. Atypical Femoral Fractures
Atypical subtrochanteric and diaphyseal femoral fractures have been reported in patients receiving denosumab products. These are unusual fractures that occur in the shaft of the femur. Patients should be advised to report any new or unusual thigh, hip, or groin pain, as this may be a sign of an impending fracture. These fractures often occur with minimal or no trauma. The exact mechanism is not fully understood but is thought to be related to the profound suppression of bone turnover, which may impair the bone’s ability to repair microdamage. The duration of therapy with Denosumab-mobz may also be a contributing factor to this rare but serious complication.
4. Multiple Vertebral Fractures (MVF) Following Discontinuation
Following the discontinuation of treatment with denosumab, there is an increased risk of MVF, particularly in patients who have had a fracture or have osteoporosis. This is a phenomenon known as the “rebound effect,” where the sudden cessation of RANKL inhibition leads to a surge in osteoclast activity and rapid bone loss. Patients should be strongly advised not to interrupt therapy with Denosumab-mobz without consulting their physician. If treatment with Denosumab-mobz is discontinued, a transition to an alternative anti-resorptive agent may be necessary to mitigate the risk of MVF.
5. Hypersensitivity
Clinically significant hypersensitivity reactions, including anaphylaxis, can occur. Patients with a known history of systemic hypersensitivity to denosumab products should not receive Denosumab-mobz. These reactions can occur within minutes of administration and require immediate medical intervention.
Common Adverse Reactions
The overall safety profile of Denosumab-mobz is consistent with that of the reference product, which is expected for a biosimilar. The most common adverse reactions reported in clinical trials vary depending on the indication.
| Indication | Common Adverse Reactions (Incidence > 10%) |
|---|---|
| Bone Metastasis from Solid Tumors | Fatigue/asthenia, hypophosphatemia, and nausea. |
| Multiple Myeloma | Diarrhea, nausea, anemia, back pain, thrombocytopenia, peripheral edema, hypocalcemia, upper respiratory tract infection, rash, and headache. |
| Giant Cell Tumor of Bone | Arthralgia, headache, nausea, back pain, fatigue, and pain in extremity. |
| Hypercalcemia of Malignancy | Nausea, vomiting, anemia, constipation, and diarrhea. |
The management of these common side effects is typically supportive, but their presence should be monitored closely, especially in the context of a patient’s underlying disease. The consistent safety data across the different indications for Denosumab-mobz underscores its predictable pharmacological profile.
Drug Interaction Profile
The drug interaction profile of Denosumab-mobz is relatively straightforward, primarily due to its nature as a monoclonal antibody. Unlike small-molecule drugs that are often metabolized by the cytochrome P450 enzyme system in the liver, biologic agents like Denosumab-mobz are typically cleared from the body through protein catabolism, minimizing the risk of pharmacokinetic drug-drug interactions. This is a major advantage in managing complex patient populations who are often on multiple medications.
Concomitant Use Warning
The most critical warning regarding drug interactions is the prohibition of concomitant use with other denosumab products. Patients receiving Denosumab-mobz should not receive other denosumab products, such as Prolia or Xgeva, at the same time. This is because all these products contain the same active ingredient (denosumab) and using them together would increase the risk of adverse effects without providing additional therapeutic benefit. This is a fundamental safety principle when prescribing Denosumab-mobz.
Formal Interaction Studies and Theoretical Concerns
The official prescribing information for Denosumab-mobz (Oziltus) states that no formal drug-drug interaction trials have been conducted with denosumab . This is a common finding for monoclonal antibodies, as their clearance mechanisms are less prone to drug-drug interactions than those of small-molecule drugs.
However, based on the mechanism of action and clinical experience with the reference product, certain theoretical or observed interactions should be considered:
- Immunosuppressive Agents: Since denosumab products can affect the immune system, caution is advised when co-administering with other immunosuppressive agents. The RANKL pathway is involved in immune cell function, and while the primary effect of Denosumab-mobz is on osteoclasts, there is a theoretical risk of increased infection when combined with other immunosuppressants. This requires careful clinical judgment and patient monitoring.
- Other Agents Affecting Bone Metabolism: The concomitant use of Denosumab-mobz with other agents that affect bone metabolism, such as bisphosphonates, is generally not recommended due to the potential for additive effects and increased risk of adverse events like ONJ. Bisphosphonates also suppress bone resorption, and combining them with Denosumab-mobz could lead to an excessive and potentially harmful reduction in bone turnover.
- Thalidomide and Bortezomib: In clinical trials for multiple myeloma, denosumab was studied in combination with standard anti-myeloma therapies, including thalidomide and bortezomib. No evidence of altered pharmacokinetics or pharmacodynamics was found, suggesting that Denosumab-mobz can be safely integrated into complex cancer treatment regimens.
In summary, while the risk of classic drug-drug interactions is low, the main concern lies in avoiding the simultaneous use of other denosumab-containing products and exercising caution with other drugs that impact bone health or the immune system. The safety and efficacy of Denosumab-mobz must always be considered in the context of a patient’s entire medication regimen.
The Biosimilar Advantage and Future of Denosumab-mobz
The approval of Denosumab-mobz marks a significant milestone in the biosimilar market. Biosimilars are not generic drugs; they are complex biological products that must demonstrate no clinically meaningful differences from the reference product. The rigorous testing and clinical trials that led to the approval of Denosumab-mobz confirm its therapeutic equivalence and safety profile. This regulatory success is a strong signal to the healthcare community about the reliability of this new treatment option.
The availability of Denosumab-mobz provides a much-needed competitive option in the treatment landscape for skeletal complications. For health systems and patients, this can translate into substantial cost savings, improving the overall sustainability of care. The confidence in the biosimilar designation means that switching from the reference product to Denosumab-mobz can be done with assurance of comparable clinical outcomes. This is particularly important in chronic disease management, where the cost of therapy can be a major barrier to adherence. The introduction of Denosumab-mobz is a direct response to the need for more affordable biologic treatments.
The continued use and study of Denosumab-mobz in real-world settings will further solidify its role. As more patients are treated with Denosumab-mobz, the long-term data will continue to reinforce the initial findings of biosimilarity. This trend is part of a broader movement in medicine to make advanced biologic therapies more accessible globally. The success of Denosumab-mobz paves the way for future biosimilar development, ensuring that innovation in biotechnology benefits a wider population. Furthermore, the competition introduced by Denosumab-mobz encourages the original manufacturer to innovate and potentially lower their prices, creating a win-win situation for the entire healthcare ecosystem. The long-term impact of Denosumab-mobz on patient care and pharmaceutical economics is expected to be profound.
Conclusion:
Denosumab-mobz is more than just a new drug; it is a testament to the progress in biosimilar development and a critical addition to the therapeutic arsenal against bone diseases. Its precise mechanism of action—the inhibition of RANKL—provides a powerful tool for preventing skeletal-related events in cancer patients and treating other serious bone conditions. The detailed understanding of its MOA, which targets the fundamental process of osteoclast-mediated bone resorption, is key to appreciating its clinical efficacy.
As a biosimilar, Denosumab-mobz offers the same high standard of efficacy and safety as the reference product, but with the added benefit of increased market competition. While the drug is highly effective, awareness of its serious side effects, such as hypocalcemia and ONJ, and the critical warning against concomitant use with other denosumab products, is essential for safe administration. The introduction of Denosumab-mobz is a positive development for patients and healthcare systems, promising both clinical excellence and greater affordability in the management of complex bone disorders. The future of biologic therapy is here, and Denosumab-mobz is leading the charge. The comprehensive data supporting Denosumab-mobz ensures that it will be a trusted and widely used option for years to come.

