Table of Contents
Introduction
Multiple myeloma is a plasma cell malignancy characterized by uncontrolled monoclonal expansion in the bone marrow, leading to anemia, bone lesions, hypercalcemia, renal dysfunction, and immunosuppression. Over the past decade, targeted immunotherapies have dramatically improved outcomes. Among them, understanding the darzalex faspro mechanism of action is essential for appreciating how modern oncology has shifted toward immune-mediated tumor eradication.
Darzalex Faspro is the subcutaneous formulation of daratumumab combined with recombinant human hyaluronidase. It provides the same therapeutic antibody exposure as the intravenous formulation while improving patient convenience and reducing infusion-related complications.
The importance of the darzalex faspro lies in its ability to harness multiple immune pathways simultaneously, creating a powerful anti-myeloma effect.
Understanding CD38: The Core Target
To fully appreciate the darzalex faspro mechanism of action, we must first understand CD38 biology.
CD38 is:
- A type II transmembrane glycoprotein
- Highly expressed on multiple myeloma cells
- Involved in NAD+ metabolism
- Associated with cell adhesion and signaling
In malignant plasma cells, CD38 expression is markedly elevated, making it an ideal immunologic target. The darzalex faspro mechanism of action centers around this overexpression.
Step-by-Step Breakdown of the Darzalex Faspro Mechanism of Action
The darzalex faspro mechanism of action operates through five interconnected immune-mediated pathways.
1. Complement-Dependent Cytotoxicity (CDC)
One major component of the darzalex faspro mechanism of action is complement activation.
When daratumumab binds to CD38:
- The Fc region recruits C1q
- The classical complement pathway is activated
- C3 convertase forms
- Membrane attack complex (MAC) forms
- Myeloma cell membrane disruption occurs
This results in direct tumor cell lysis.
CDC effectiveness may vary depending on complement protein availability and tumor microenvironment factors.
2. Antibody-Dependent Cellular Cytotoxicity (ADCC)
Another essential pathway in the darzalex faspro mechanism of action is ADCC.
Process:
- Daratumumab coats CD38-positive cells
- NK cells recognize Fc region via FcγRIIIa receptor
- NK cells release perforin and granzymes
- Target cell apoptosis occurs
Genetic polymorphisms in Fc receptors may influence ADCC intensity.
3. Antibody-Dependent Cellular Phagocytosis (ADCP)
Macrophages play a critical role in the darzalex faspro mechanism of action through:
- Fc receptor engagement
- Opsonized tumor recognition
- Phagocytic engulfment
- Lysosomal degradation
ADCP contributes significantly to tumor burden reduction within bone marrow niches.
4. Direct Apoptosis via Cross-Linking
Cross-linking of CD38 triggers:
- Intracellular calcium signaling changes
- Caspase cascade activation
- Mitochondrial dysfunction
- Programmed cell death
This direct cytotoxic pathway enhances the potency of the darzalex faspro mechanism of action independent of immune effector cells.
5. Immunomodulatory Microenvironment Remodeling
A unique aspect of the darzalex faspro mechanism of action is immune reprogramming.
It reduces:
- Regulatory T cells (Tregs)
- Regulatory B cells
- Myeloid-derived suppressor cells
Simultaneously, it enhances:
- Cytotoxic CD8+ T-cell expansion
- Interferon-gamma production
- Anti-tumor immune memory
This long-term immune remodeling extends therapeutic durability.
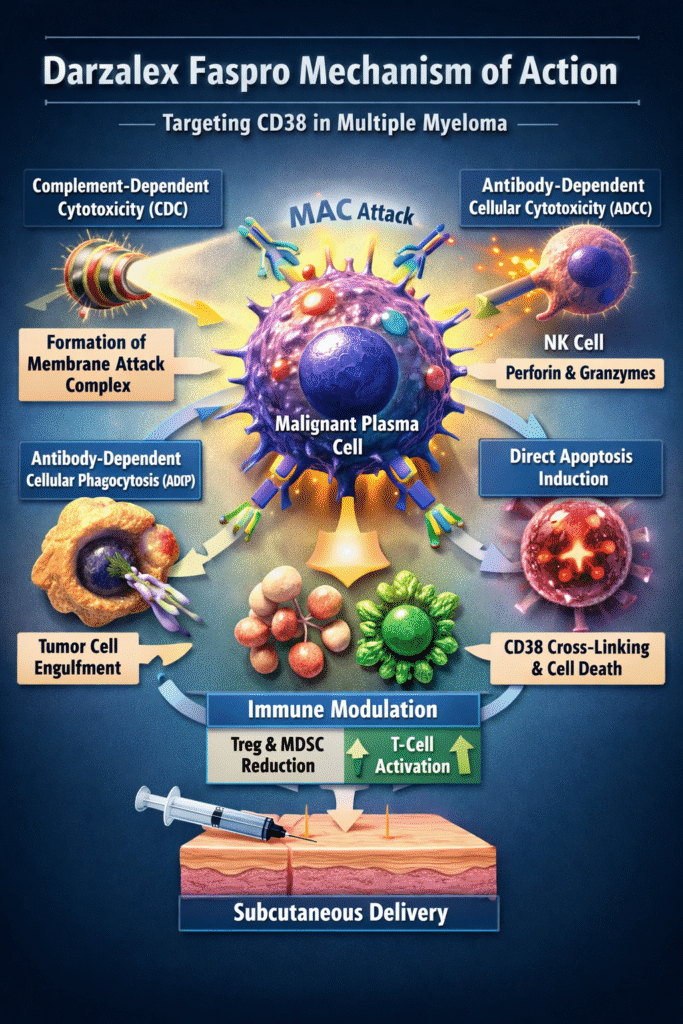
Role of Hyaluronidase in Subcutaneous Delivery
While not cytotoxic itself, hyaluronidase enables dispersion of daratumumab through subcutaneous tissue by temporarily degrading hyaluronan.
This ensures proper systemic absorption necessary for maintaining the full darzalex faspro mechanism of action.
Pharmacokinetics and Pharmacodynamics
Pharmacokinetic studies demonstrate:
- Comparable trough concentrations to IV daratumumab
- Sustained CD38 saturation
- Predictable exposure
Pharmacodynamically, the darzalex faspro mechanism of action produces rapid reductions in circulating malignant plasma cells.
FDA Approval History
Darzalex Faspro received FDA approval on29 January 2026 for:
- Newly diagnosed multiple myeloma
- Relapsed or refractory multiple myeloma
- Combination regimens in frontline and later lines
Approval was based on studies confirming non-inferiority while maintaining the identical darzalex faspro mechanism of action.
Clinical Trial Highlights
Major studies showed:
- Similar overall response rates compared to IV form
- Lower infusion-related reaction rates
- Improved patient satisfaction
The durability of response supports the strength of the darzalex faspro mechanism of action across multiple treatment lines.
Indications and Combination Therapy
Darzalex Faspro is commonly used in combination with:
- Lenalidomide + dexamethasone
- Bortezomib + melphalan + prednisone
- Carfilzomib-based regimens
These combinations enhance tumor cell stress, complementing the darzalex faspro mechanism of action.
Side Effects and Safety Profile
Because the darzalex faspro mechanism of action activates immune pathways, side effects are primarily immune-related.
Common Adverse Effects
- Injection-site reactions
- Fatigue
- Upper respiratory tract infections
- Diarrhea
- Peripheral edema
Hematologic Toxicities
- Neutropenia
- Anemia
- Thrombocytopenia
Serious Risks
- Herpes zoster reactivation
- Severe infections
- Interference with blood typing
Prophylactic antiviral therapy is often recommended.
Drug Interactions
The darzalex faspro mechanism of action can interfere with indirect antiglobulin testing because CD38 is expressed on red blood cells.
Important considerations:
- Inform blood banks before transfusion
- Baseline blood typing recommended
- Monitor additive immunosuppression in combination regimens
Mechanisms of Resistance
Despite strong efficacy, resistance may occur due to:
- Downregulation of CD38 expression
- Upregulation of complement inhibitory proteins
- Bone marrow stromal protection
- NK cell exhaustion
Understanding these mechanisms helps preserve the clinical impact of the darzalex faspro mechanism of action.
Comparison With Other CD38 Antibodies
Isatuximab is another CD38-targeting antibody. However, subtle differences exist in binding epitopes and immune activation intensity.
Still, the established clinical track record of the darzalex faspro mechanism of action makes it a standard of care in many regimens.
Benefits of Subcutaneous Formulation
Advantages include:
- 3–5 minute administration
- Reduced chair time
- Lower infusion reaction rates
- Improved patient convenience
These benefits increase accessibility while preserving the darzalex faspro mechanism of action.
Real-World Effectiveness
Real-world studies show:
- High overall response rates
- Improved progression-free survival
- Acceptable safety in elderly populations
The robustness of the darzalex faspro mechanism of action extends beyond clinical trial settings.
Future Directions
Ongoing research explores:
- Earlier use in smoldering myeloma
- Combination with CAR-T therapy
- Combination with bispecific antibodies
- Minimal residual disease–guided treatment
These innovations aim to maximize the long-term potential of the darzalex faspro mechanism of action.
Conclusion
The darzalex faspro mechanism of action represents one of the most powerful targeted immunologic strategies in hematologic oncology. By combining complement activation, antibody-dependent cytotoxicity, macrophage-mediated phagocytosis, apoptosis induction, and immune microenvironment remodeling, it achieves comprehensive tumor eradication.
With strong FDA approval support, manageable safety profile, and convenient subcutaneous delivery, Darzalex Faspro continues to redefine multiple myeloma therapy.
Understanding the darzalex faspro mechanism of action is essential for clinicians, pharmacists, and researchers aiming to optimize patient outcomes and explore next-generation immunotherapy combinations.