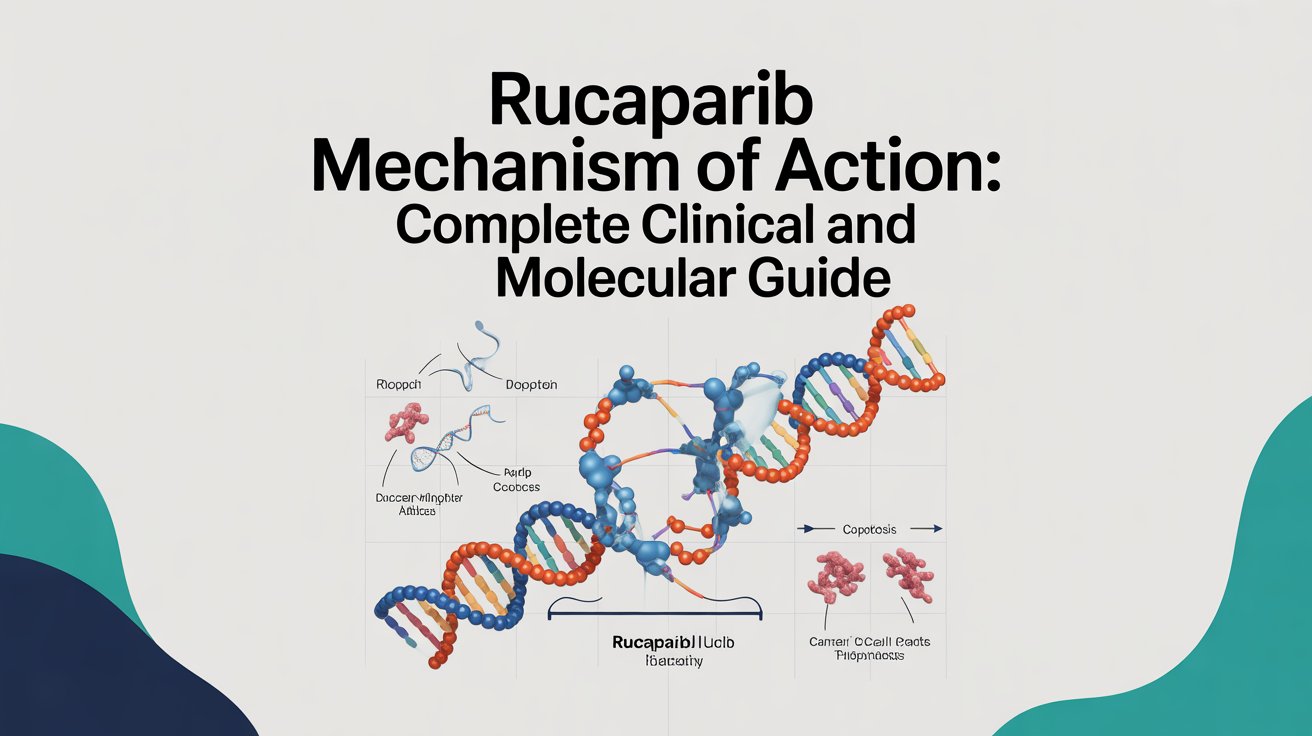
Rucaparib Mechanism of Action: Complete Clinical and Molecular Guide
Introduction Targeted cancer therapy has transformed modern oncology by shifting treatment strategies from non-specific cytotoxic chemotherapy to precision-driven molecular inhibition. One of the most significant advancements in this space is the development of PARP inhibitors, including rucaparib. Understanding the rucaparib mechanism of action is essential for healthcare professionals, pharmacists, oncology researchers, and students who want …