Table of Contents
Introduction
Acquired thrombotic thrombocytopenic purpura (aTTP) is a rare, life-threatening hematologic disorder characterized by widespread microvascular thrombosis, severe thrombocytopenia, and organ ischemia. Before the introduction of targeted biologics, mortality rates were significantly high despite plasma exchange therapy. The development of caplacizumab (marketed as Cablivi) marked a breakthrough in the management of aTTP.
However, as with any targeted therapy affecting coagulation pathways, understanding cablivi side effects is critical for ensuring safe and effective treatment. While Cablivi dramatically reduces clot formation and improves platelet recovery time, it also increases bleeding risk due to its direct effect on von Willebrand factor (vWF).
This comprehensive, authority-level article explores everything you need to know about :
- Mechanism of action (MOA)
- FDA approval background
- Clinical trial safety data
- Common and serious adverse reactions
- Bleeding risk management
- Drug interactions
- Monitoring recommendations
- Special population considerations
- Patient counseling strategies
- Long-term safety insights
Whether you are a healthcare professional, pharmacist, medical student, or patient seeking clarity, this guide provides in-depth clarity about cablivi side effects and how to manage them effectively.
Understanding Cablivi: Overview and Therapeutic Role
Cablivi (caplacizumab-yhdp) is a humanized, bivalent nanobody designed to target the A1 domain of von Willebrand factor. It is approved for use in adults with acquired thrombotic thrombocytopenic purpura in combination with plasma exchange and immunosuppressive therapy.
Why Is Cablivi Needed in aTTP?
aTTP results from severe deficiency of ADAMTS13 enzyme activity, typically due to autoantibodies. Without ADAMTS13:
- Ultra-large vWF multimers accumulate
- Platelets bind excessively
- Microthrombi form in small blood vessels
- Organ damage occurs
Cablivi interrupts this process rapidly. However, the same mechanism responsible for therapeutic success also explains many cablivi side effects, especially bleeding events.
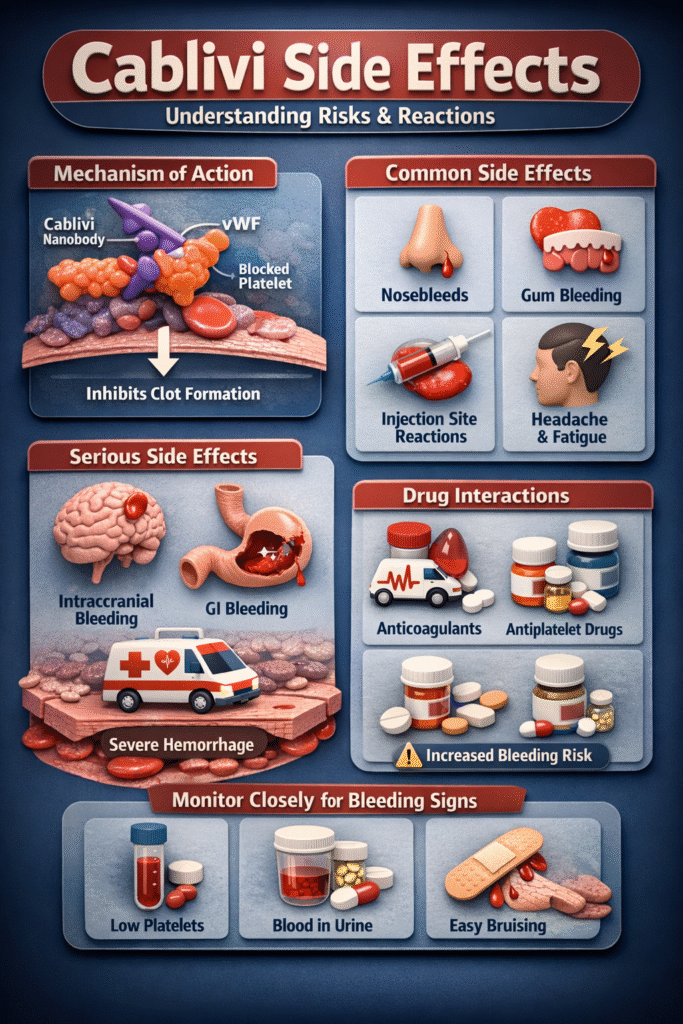
Mechanism of Action (MOA)
To fully understand cablivi side effects, we must examine how the drug works at a molecular level.
Step 1: Targeting von Willebrand Factor
Cablivi binds selectively to the A1 domain of vWF.
Step 2: Blocking Platelet Adhesion
Normally, vWF interacts with platelet glycoprotein Ib receptors to promote clot formation. Cablivi blocks this interaction.
Step 3: Preventing Microthrombi
By inhibiting platelet adhesion, Cablivi prevents the formation of microvascular clots.
Step 4: Rapid Platelet Recovery
Platelet consumption decreases, leading to normalization of platelet counts.
How MOA Leads to Side effects of Cablivi
Because Cablivi suppresses platelet adhesion:
- Hemostasis is partially impaired
- Bleeding time increases
- Minor injuries may bleed longer
This explains why bleeding is the primary category of cablivi side effects.
FDA Approval and Clinical Trial Evidence
Cablivi received FDA approval in 2019 following pivotal clinical trials demonstrating:
- Faster time to platelet count normalization
- Reduced recurrence of TTP
- Reduced composite outcomes (death, recurrence, thromboembolism)
- Shorter hospital stay duration
However, safety data from these trials highlighted predictable cablivi side effects, particularly mucocutaneous bleeding.
Post-marketing surveillance continues to monitor the safety profile to better understand long-term side effects of cablivi.
Comprehensive Review of Cablivi Side Effects
1. Common Cablivi Side Effects
The most frequently reported cablivi side effects include:
- Epistaxis (nosebleeds)
- Gingival bleeding
- Injection site reactions
- Headache
- Fatigue
- Bruising
- Mild gastrointestinal bleeding
Most of these are mild to moderate in severity and manageable.
2. Bleeding: The Most Important Cablivi Side Effect
Bleeding represents the most clinically significant among all side effects of cablivi.
Types of Bleeding Observed
- Nosebleeds
- Gum bleeding
- Vaginal bleeding
- Hematuria
- Gastrointestinal bleeding
In rare cases:
- Intracranial hemorrhage
- Severe gastrointestinal hemorrhage
The risk increases significantly when combined with anticoagulants.
3. Serious Cablivi Side Effects
Although uncommon, serious side effects of cablivi may include:
- Major hemorrhage requiring hospitalization
- Anaphylaxis
- Severe hypersensitivity reactions
- Severe thrombocytopenia rebound after discontinuation
Immediate medical intervention is required in such cases.
4. Injection Site Reactions
Subcutaneous administration may lead to:
- Pain
- Swelling
- Redness
- Pruritus
These localized cablivi side effects are usually mild and self-limiting.
5. Allergic Reactions
Hypersensitivity is a rare but documented category of side effects of cablivi, presenting as:
- Rash
- Urticaria
- Dyspnea
- Hypotension
Discontinuation is necessary in severe reactions.
Risk Factors That Increase side effects of cablivi
Certain populations are more vulnerable:
- Patients on anticoagulants
- Patients on antiplatelet therapy
- Liver disease patients
- Recent surgical patients
- Individuals with bleeding disorders
Careful risk assessment reduces serious cablivi side effects.
Drug Interactions
Drug interactions significantly amplify cablivi side effects.
- Warfarin
- Heparin
- Apixaban
- Rivaroxaban
Combination increases hemorrhagic risk.
Antiplatelet Agents
- Aspirin
- Clopidogrel
- Ticagrelor
May intensify bleeding-related cablivi side effects.
NSAIDs
Ibuprofen and similar drugs may worsen bleeding tendencies.
Clinical vigilance is necessary to avoid compounded cablivi side effects.
Monitoring Recommendations
To minimize cablivi side effects, healthcare providers monitor:
- Platelet counts
- Signs of active bleeding
- Hemoglobin levels
- Coagulation parameters
Patient self-monitoring is equally important.
Management Strategies for side effects of cablivi
Mild Bleeding
- Continue therapy with monitoring
- Avoid NSAIDs
Moderate Bleeding
- Temporary interruption
- Supportive care
Severe Bleeding
- Immediate discontinuation
- Administer vWF concentrate
- Hemodynamic stabilization
Timely intervention prevents escalation of cablivi side effects.
Special Populations
Pregnancy
Limited safety data available. Bleeding risk must be carefully considered when evaluating potential cablivi side effects.
Elderly
Age-related hemostatic changes may increase susceptibility to cablivi side effects.
Hepatic Impairment
Liver dysfunction may increase bleeding risk.
Renal Impairment
Requires close monitoring for worsening cablivi side effects.
Long-Term Safety and Real-World Evidence
Long-term extension studies indicate:
- Most cablivi side effects occur early during treatment
- Severe bleeding remains uncommon with appropriate monitoring
- Risk decreases after treatment completion
Ongoing pharmacovigilance ensures early detection of emerging safety concerns.
Benefits Versus Risks
Despite the concern regarding cablivi side effects, benefits include:
- Reduced mortality
- Faster disease control
- Lower relapse rates
- Reduced organ damage
The therapeutic advantage outweighs risks when managed appropriately.
Patient Counseling Guide
Patients should be instructed to:
- Avoid contact sports
- Report unusual bruising
- Inform doctors before surgery
- Avoid OTC NSAIDs
- Watch for signs of internal bleeding
Proper education minimizes preventable cablivi side effects.
Clinical Pearls for Healthcare Professionals
- Always assess bleeding risk before initiation
- Avoid concurrent anticoagulation when possible
- Monitor platelet recovery carefully
- Educate patients thoroughly
Clinical awareness significantly reduces complications from side effects of cablivi.
Future Perspectives
Emerging research aims to:
- Optimize dosing strategies
- Reduce bleeding risk
- Improve long-term outcomes
- Identify biomarkers predicting side effects of cablivi
Personalized medicine may further enhance safety profiles.
Conclusion
Cablivi has revolutionized the management of acquired thrombotic thrombocytopenic purpura. By specifically targeting von Willebrand factor, it interrupts the pathologic clotting cascade and dramatically improves patient outcomes.
However, its mechanism inherently increases bleeding risk, making awareness of cablivi side effects essential. Most adverse reactions are mild and manageable, but serious hemorrhage can occur, particularly when combined with anticoagulants or in high-risk populations.
Through careful monitoring, appropriate drug interaction management, and thorough patient education, healthcare providers can maximize therapeutic benefits while minimizing side effects of cablivi.
Ultimately, informed clinical decision-making ensures that patients receive life-saving treatment safely and effectively.

