Table of Contents
Bone health is one of the most important aspects of cancer treatment, especially for people whose disease has spread to the skeleton. Older cancer patients often face painful and dangerous bone complications, such as fractures, spinal cord compression, and bone lesions that require radiation or surgery. Bomyntra is a newly approved treatment designed to prevent these skeletal-related events.
First approved by the US Food and Drug Administration (FDA) on March 25, 2025, Bomyntra (denosumab-BNHT) is biosimilar to Xgeva (denosumab) and belongs to the class of RANK ligand (RANKL) inhibitors. This blog post will cover everything you need to know about Bomyntra, including its approval, mechanism of action, benefits, indications, side effects, and frequently asked questions.
What is Bomyntra?
Bomyntra is a prescription medicine that prevents bone complications in adults with advanced cancer that has spread to the bones. The medicine is specifically designed to manage skeletal-related events (SREs) such as:
• Bone fractures (broken bones)
• Spinal cord compression (pressure on the spinal cord due to weakened bones)
• Bone problems that require radiation therapy
• Bone damage that requires surgery
Bomyntra is a biosimilar version of denosumab, which means it is the same as an already approved biologic medicine (Xgeva®) but is developed by a different manufacturer with no clinically meaningful differences in safety, quality, or effectiveness.
FDA Approval of Bomyntra
On March 25, 2025, the FDA approved Bomyntra, marking a milestone in cancer care. Its approval provides patients and healthcare systems with a cost-effective alternative to branded Xgeva while providing similar clinical benefits.
Biosimilar of Xgeva is indicated for the following conditions:
1. Prevention of skeletal-related events in patients with multiple myeloma.
2. Prevention of skeletal complications in patients with solid tumors that have metastasized to bone.
3. Treatment of giant cell tumor of bone (GCTB) in adults and adolescents (with fully mature skeletons).
4. Treatment of hypercalcemia of malignancy (HCM), especially when patients have had an inadequate response to bisphosphonates.
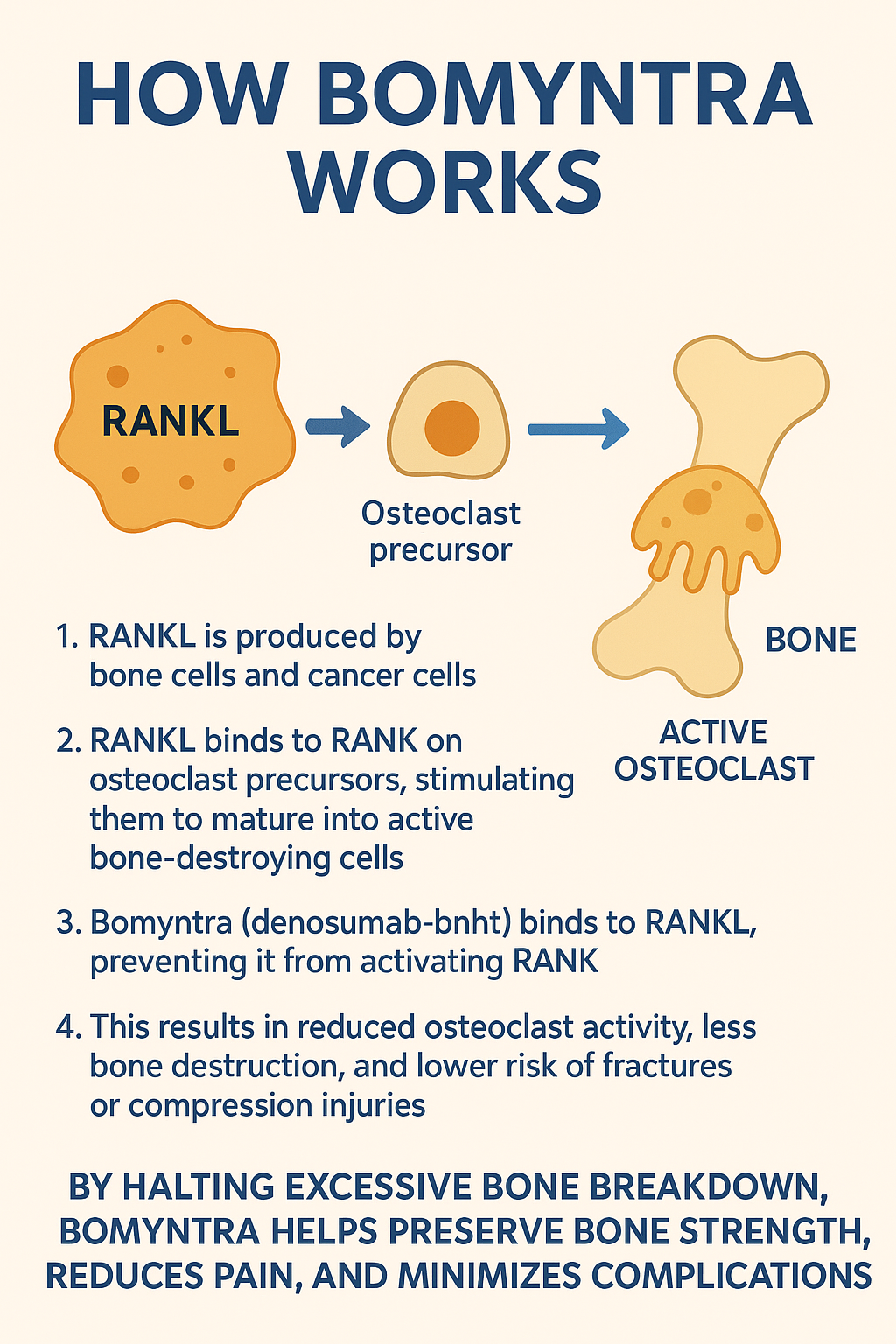
Mechanism of Action
To understand Bomyntra, we must first understand bone metabolism in cancer.
- Bones are constantly broken down and rebuilt by cells known as osteoclasts (bone-resorbing cells) and osteoblasts (bone-forming cells).
- In cancer, especially when tumors spread to the bone, osteoclast activity often becomes overactive, leading to weakened bones, fractures, and pain.
- Bomyntra works by blocking RANK ligand (RANKL), a protein that activates osteoclasts.
Step-by-Step Mechanism:
- RANKL is produced by bone cells and cancer cells.
- RANKL binds to RANK on osteoclast precursors, stimulating them to mature into active bone-destroying cells.
- Bomyntra (denosumab-bnht) binds to RANKL, preventing it from activating RANK.
- This results in reduced osteoclast activity, less bone destruction, and lower risk of fractures or compression injuries.
By halting excessive bone breakdown, Biosimilar of Xgeva helps preserve bone strength, reduces pain, and minimizes complications.
Benefits
Bomyntra offers several important benefits for patients with advanced cancer and bone involvement:
- Prevents Bone Fractures – Reduces risk of painful, debilitating bone breaks.
- Lowers Risk of Spinal Cord Compression – Protects the spinal cord from damage caused by weakened vertebrae.
- Reduces Need for Surgery or Radiation – Decreases bone-related interventions, improving quality of life.
- Effective in Multiple Cancers – Useful in multiple myeloma and solid tumors (such as breast, prostate, or lung cancer) with bone metastases.
- Alternative for Bisphosphonate-Resistant Patients – Effective even when standard therapies like zoledronic acid fail.
- Treats Giant Cell Tumor of Bone – Provides non-surgical treatment for rare bone tumors.
- Controls Hypercalcemia of Malignancy – Helps normalize dangerously high calcium levels in cancer patients.
Dosage and Administration
The recommended dosage of Bomyntra depends on the condition being treated. It is given as a subcutaneous (under the skin) injection by a healthcare provider.
- Multiple Myeloma / Solid Tumors with Bone Metastases:
120 mg once every 4 weeks. - Giant Cell Tumor of Bone (Adults & Adolescents):
120 mg every 4 weeks, with additional doses on days 8 and 15 of the first month. - Hypercalcemia of Malignancy:
120 mg every 4 weeks, with possible additional doses depending on calcium levels.
Patients may also be prescribed calcium and vitamin D supplements to reduce the risk of low calcium levels (hypocalcemia).
Side Effects for Biosimilar of Xgeva
Like all medications, Bomyntra can cause side effects. While most are mild to moderate, some may be serious.
Common Side Effects:
- Fatigue
- Nausea
- Low calcium levels (hypocalcemia)
- Joint and muscle pain
- Headache
Serious Side Effects:
- Osteonecrosis of the jaw (ONJ) – A rare but serious condition where the jawbone does not heal properly.
- Severe hypocalcemia – Can cause muscle spasms, seizures, or heart rhythm problems.
- Serious infections – Since it affects the immune system, there is a slight risk of infections.
- Skin reactions – Rash, dermatitis, or eczema.
Patients should be closely monitored during treatment, especially for calcium levels and dental health.
Bomyntra vs. Xgeva
Since Bomyntra is a biosimilar to Xgeva, many patients wonder if there are differences between the two.
| Feature | Xgeva (Original) | Bomyntra (Biosimilar) |
| Active Ingredient | Denosumab | Denosumab-bnht |
| Manufacturer | Amgen | Another biosimilar company |
| Approval | Earlier | March 25, 2025 |
| Efficacy | Same | Same |
| Safety | Same | Same |
| Cost | Higher | Lower, more affordable |
Bomyntra provides the same clinical benefits as Xgeva but at a reduced cost, making it more accessible for patients and healthcare providers.
Who Should Not Use Biosimilar of Xgeva ?
Bomyntra may not be suitable for all patients. It should be avoided in:
- Patients with severe hypocalcemia (very low calcium levels).
- Individuals allergic to denosumab or any of its ingredients.
- Pregnant or breastfeeding women, unless clearly prescribed by a doctor.
Precautions and Monitoring
Patients receiving Biosimilar of Xgeva should follow certain precautions:
- Calcium & Vitamin D supplements must be taken regularly.
- Dental checkups are recommended before starting treatment to reduce the risk of jawbone problems.
- Blood calcium levels should be monitored during therapy.
- Inform your doctor about other medications (bisphosphonates, steroids, chemotherapy).
Future of Biosimilar of Xgeva in Cancer Treatment
The approval of Biosimilar of Xgeva represents a major step forward in biosimilar adoption. By reducing costs while maintaining clinical effectiveness, Biosimilar of Xgeva is expected to expand access to critical supportive care in oncology.
As more biosimilars like Biosimilar of Xgeva enter the market, patients will benefit from affordable and effective treatment options that improve quality of life and survival outcomes.
Conclusion
Bomyntra(denosumab-BNHT) is a groundbreaking FDA-approved biosimilar that provides a reliable and cost-effective way to prevent bone complications in cancer patients. By targeting the RANKL pathway, it effectively reduces skeletal-related events such as fractures, spinal compression, and the need for radiation or surgery. With approval on March 25, 2025, Bomyntra is poised to improve access to essential cancer care while reducing financial burden. As a safer, more effective, and more affordable alternative to Xgeva, it represents a powerful step forward in oncology treatment.

1 thought on “Bomyntra: A Complete Guide to the RANKL Inhibitor for Bone Complications in Cancer Patients”