- Brand Name : Blujepa
- Generic Name : gepotidacin
- Drug Class: Triazaacenaphthylene antibiotic
- Mechanism: Bacterial type II topoisomerase inhibitor
- Indication: Acute uncomplicated and recurrent UTIs
- Approval Date: March 2025
- Formulation: Oral tablets (750 mg)
Table of Contents
Introduction :
Urinary tract infections (UTIs) are the most common bacterial infections in women. With increasing antibiotic resistance, the medical community urgently needs new, more effective agents. In a major breakthrough, the US FDA approved Blujepa in March 2025 for the treatment of uncomplicated urinary tract infections (uUTIs) in female patients aged 12 years and older. Blujepa 750 mg tablets, taken twice daily for 5 days, offer targeted action with a novel mechanism of action.
Let’s explore the full potential of Blujepa, a triazenaphtheline-based bacterial type II topoisomerase inhibitor, which is shaping the treatment of UTIs.
What is Blujepa?
Blujepa is a first-in-class antibiotic of the triazenaphthalene family. It works by inhibiting bacterial type II topoisomerases – DNA gyrase and topoisomerase IV – which are enzymes important for bacterial DNA replication. This makes Blujepa particularly effective against multidrug-resistant (MDR) uropathogens.
✅ FDA-Approved Indication :
Blujepa(gepotidacin) is approved for:
- Uncomplicated urinary tract infections (uUTIs) in
- Female adult and pediatric patients ≥12 years old
- Weighing ≥88 lbs (40 kg)
- Caused by Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii complex, Staphylococcus saprophyticus, and Enterococcus faecalis
⚠️ Important: It should only be used to treat infections proven or strongly suspected to be bacterial in origin, to help prevent the spread of antibiotic resistance.
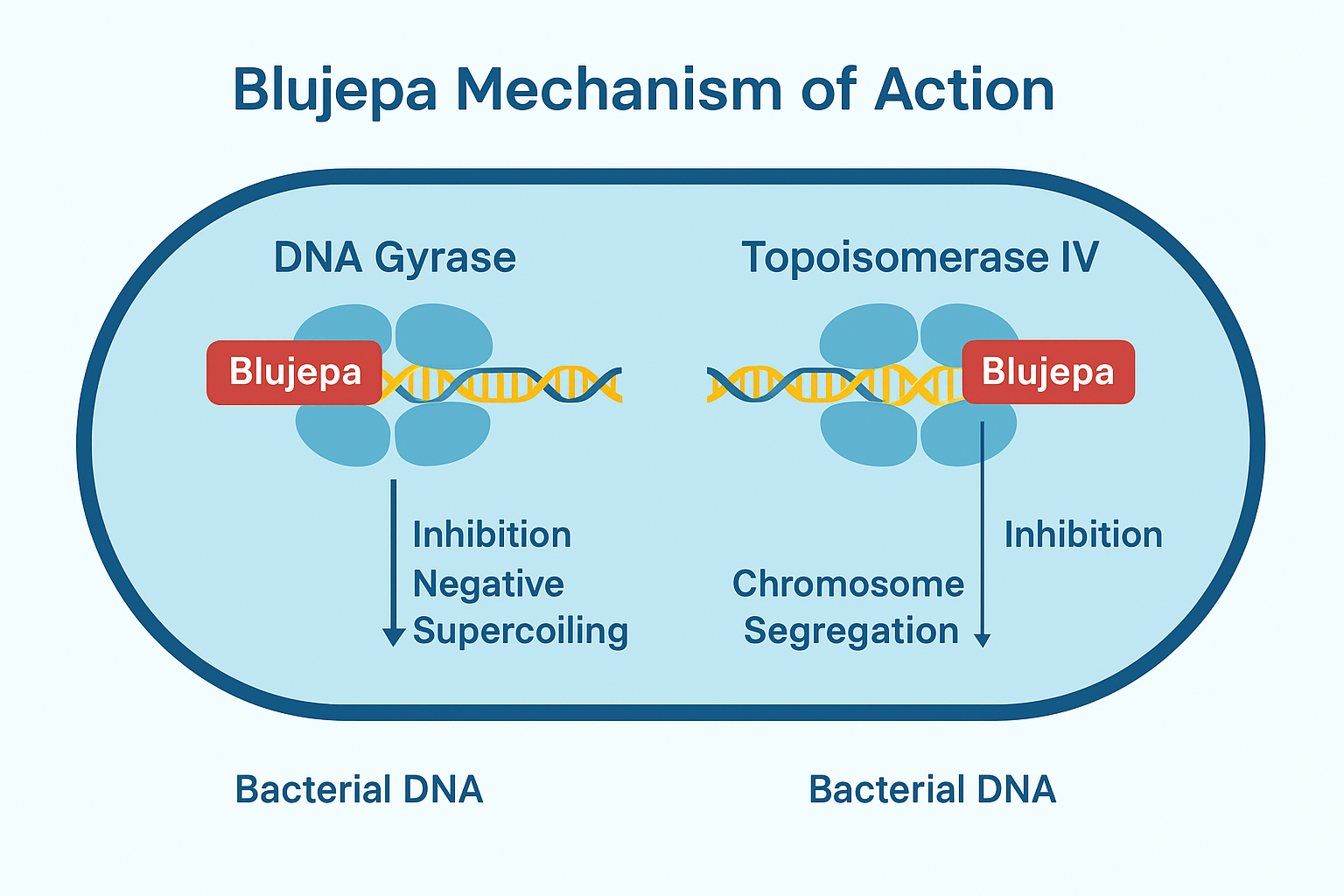
Mechanism of Action :
This antibiotic is a bacterial type II topoisomerase inhibitor that targets both:
- DNA Gyrase (GyrA/GyrB) – disrupts supercoiling required for replication
- Topoisomerase IV (ParC/ParE) – interferes with chromosome segregation
💡 It binds at novel, non-quinolone sites, so it avoids cross-resistance seen with fluoroquinolones.
Why gepotidacin is a Game-Changer ?
Due to its unique structure and mechanism, Blujepa:
- Targets MDR pathogens
- Works in adolescents and adults
- Has minimal impact on gut microbiota
- Offers a short, 5-day treatment regimen with convenient oral dosing
🔬 Clinical data shows:
- 90% microbiological cure
- Faster symptom relief than comparators
- Excellent safety profile across all ages
Dosage and Administration :
| Patient Population | Dosage | Duration |
| Females ≥12 years old and ≥88 lbs (40 kg) | 750 mg tablet orally twice daily | 5 days |
- Form: Oral tablet
- Take with or without food
- Do not take with antacids, iron, or magnesium within 2 hours
💊It is supplied as 750 mg tablets, ensuring easy administration and high patient compliance.
Pathogens Targeted by Blujepa :
Blujepa is approved for infections caused by:
- Escherichia coli
- Klebsiella pneumoniae
- Citrobacter freundii complex
- Staphylococcus saprophyticus
- Enterococcus faecalis
These organisms are responsible for the majority of uUTIs, particularly in females.
Clinical Trials Overview :
In Phase 3 trials involving over 2,000 participants:
- Microbiological eradication rate: >90%
- Symptom relief in <48 hours for most patients
- Low relapse rate: <7% within 30 days
- Comparable or superior efficacy to nitrofurantoin and TMP-SMX
These results were pivotal in the FDA’s March 2025 approval.
Side Effects :
Most patients tolerate Blujepa well. Reported side effects are mild to moderate and resolve quickly.
Common Side Effects:
- Headache
- Nausea
- Diarrhea
- Fever
- Dizziness
Less Common:
- Mild skin rash
- Elevated liver enzymes (transient)
- Rare cases of QT prolongation
📌 ECG monitoring is advised for patients with heart rhythm disorders or those on QT-prolonging drugs.
Drug Interactions :
Use cautiously with:
- QT-prolonging drugs (e.g., amiodarone, sotalol)
- Anticoagulants (e.g., warfarin)
- Magnesium/aluminum-based antacids or supplements
✅ To ensure optimal absorption, take Blujepa at least 2 hours before or after such substances.
Who Should Not Use gepotidacin?
- Patients with known hypersensitivity to triazaacenaphthylene compounds
- Females under 12 years of age
- Those under 40 kg (88 lbs)
- Patients at high risk of QT interval prolongation
Storage Instructions :
- Store at 25°C (77°F)
- Keep in original blister pack or bottle
- Protect from light and moisture
- Keep out of reach of children
| Feature | Blujepa (2025) | Nitrofurantoin / TMP-SMX |
| Drug Class | Triazaacenaphthylene | Nitrofuran, sulfonamide combo |
| Resistance Profile | Active against MDR pathogens | Increasing resistance |
| Dosage | 750 mg BID for 5 days | Varies, longer duration |
| Mechanism of Action | Topoisomerase II inhibitor | Various |
| Age Approved | ≥12 years, ≥88 lbs | Adults (off-label for kids) |
Conclusion
Blujepa 750 mg tablets represent a new era in the treatment of uncomplicated urinary tract infections in women. As the first triazacenaphthalene bacterial type II topoisomerase inhibitor, it provides potent, fast-acting, and targeted treatment against particularly resistant pathogens. With a short 5-day course, low resistance potential, and FDA support,it is poised to become a mainstay in women’s urological health.
Used responsibly, It not only treats infections but also helps prevent the emergence of antibiotic resistance – a global health priority.
Frequently Asked Questions (FAQs)
Q1. What is Blujepa used for?
Q2. How should I take gepotidacin?
Q3. Is Blujepa effective against drug-resistant bacteria?
Q4. Can men use gepotidacin ?
Q5. Can I take gepotidacin if I’m pregnant?
Disclaimer: This blog post is for informational purposes only and does not constitute medical advice. Always consult a qualified healthcare professional before making any medical decisions.

1 thought on “Blujepa: FDA-Approved Topoisomerase Inhibitor for Uncomplicated UTIs in Females (2025)”