Table of Contents
Introduction
Immunotherapy has transformed the landscape of cancer treatment, especially in hematologic malignancies where traditional chemotherapy often falls short. One of the most groundbreaking innovations in this space is Blincyto (blinatumomab). The Blincyto mechanism of action represents a paradigm shift from non-specific cytotoxic therapy to precise immune-directed tumor killing.
Blincyto is the first FDA-approved bispecific T-cell engager (BiTE) antibody, specifically designed to redirect the patient’s own immune system to destroy malignant B-cells. Unlike chemotherapy, which indiscriminately damages rapidly dividing cells, the Blincyto mechanism of action selectively targets CD19-expressing leukemia cells by activating cytotoxic T-cells.
Understanding the Blincyto mechanism of action is essential for clinicians, pharmacists, researchers, and students seeking insight into modern immuno-oncology. This article provides a deep scientific and clinical exploration of how Blincyto works, its safety profile, FDA approval history, and important drug interaction considerations.
Overview of Blincyto (Blinatumomab)
Blincyto is a recombinant bispecific monoclonal antibody composed of two single-chain variable fragments connected by a flexible linker. These fragments bind simultaneously to:
- CD19 on B-cells
- CD3 on T-cells
This unique structure is central to the Blincyto mechanism of action, allowing direct physical linkage between immune cells and leukemia cells. Importantly, Blincyto lacks an Fc region, which reduces nonspecific immune activation and influences its pharmacokinetic behavior.
The Blincyto mechanism of action is independent of antigen presentation via major histocompatibility complex (MHC), making it effective even in tumors that evade immune detection.
Blincyto Mechanism of Action (MOA)
Core Principle of the Mechanism of Action
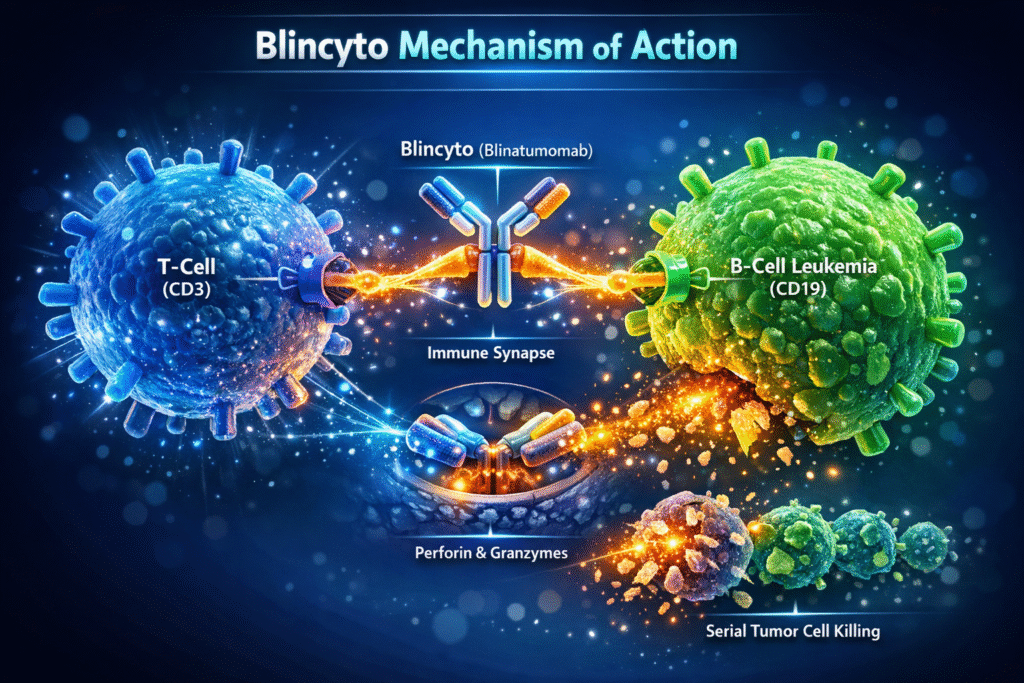
At its core, the Blincyto mechanism of action involves immune redirection. Blincyto acts as a molecular bridge that brings cytotoxic T-cells into direct contact with CD19-positive leukemia cells, triggering targeted immune-mediated cell death.
1. Dual Antigen Binding
The Blincyto mechanism of action begins with simultaneous binding to CD3 on T-cells and CD19 on B-cells. This dual binding is highly specific and does not require co-stimulatory molecules or antigen processing.
2. Immune Synapse Formation
By physically linking T-cells and leukemia cells, the Blincyto mechanism of action facilitates formation of an immune synapse. This close cellular interaction mimics natural T-cell receptor signaling and initiates downstream cytotoxic pathways.
3. T-Cell Activation and Expansion
Engagement of CD3 triggers intracellular signaling cascades within T-cells, leading to:
- T-cell activation
- Clonal expansion
- Cytokine production
The Blincyto mechanism of action can activate both naïve and memory T-cells, even in heavily pretreated patients.
4. Cytotoxic Molecule Release
Activated T-cells release perforin and granzymes, which penetrate the leukemia cell membrane and induce apoptosis. This direct killing process is a defining feature of the Blincyto mechanism of action.
5. Serial Tumor Cell Killing
One of the most powerful aspects of the Blincyto mechanism of action is its ability to enable serial killing. A single T-cell can destroy multiple leukemia cells, enhancing treatment efficiency and depth of response.
6. B-Cell Depletion
Because CD19 is expressed on normal and malignant B-cells, the Blincyto mechanism of action results in global B-cell depletion, contributing to both therapeutic efficacy and immune-related adverse effects.
Immunologic Precision of the Mechanism of Action
The Blincyto mechanism of action offers remarkable specificity compared to chemotherapy. Its compact molecular design allows rapid tissue penetration and efficient immune synapse formation, enabling strong antitumor effects at very low concentrations.
Importantly, the Blincyto mechanism of action does not rely on antigen-presenting cells, making it effective in immunocompromised environments.
Minimal Residual Disease (MRD)
One of the most clinically significant advantages of the Blincyto mechanism of action is its ability to eradicate minimal residual disease. MRD is a major cause of leukemia relapse, and Blincyto’s immune surveillance capability allows it to detect and eliminate residual malignant cells beyond the reach of conventional therapies.
Tumor Microenvironment and Immune Resistance
The leukemia microenvironment often suppresses immune activity. However, the Blincyto mechanism of action overcomes this barrier by forcing direct contact between T-cells and tumor cells, allowing cytotoxic signaling to proceed even in immunosuppressive conditions.
Side Effects Related to the Blincyto
Cytokine Release Syndrome (CRS)
CRS is a predictable consequence of the Blincyto mechanism of action due to rapid T-cell activation. Symptoms include fever, hypotension, and systemic inflammation, most commonly occurring early in therapy.
Neurologic Toxicity
Neurotoxicity associated with the Blincyto mechanism of action may include confusion, seizures, and speech disturbances. These effects are generally reversible with dose interruption and supportive care.
Infections and Immunoglobulin Depletion
Because the Blincyto mechanism of action depletes B-cells, patients may experience hypogammaglobulinemia and increased infection risk, necessitating long-term immune monitoring.
FDA Approval History
The unique Blincyto mechanism of action led to multiple regulatory milestones:
- Accelerated FDA approval for relapsed or refractory B-cell ALL
- Expanded approval for MRD-positive patients in remission
- Approval in both adult and pediatric populations
These approvals reflect strong confidence in the safety and efficacy of the Blincyto .
Drug Interactions
Immunosuppressive Agents
Drugs such as corticosteroids may reduce the effectiveness of the Blincyto mechanism of action if used excessively, although short-term use is often required to manage CRS.
Vaccines
Live vaccines should be avoided during treatment due to immune suppression caused by the Blincyto.
Other Immunotherapies
Concurrent immune-activating therapies may increase the risk of toxicity due to overlapping mechanisms with the Blincyto mechanism of action.
Administration Considerations
Due to its short half-life, Blincyto is administered as a continuous intravenous infusion. This delivery method ensures sustained immune engagement, which is essential for the Blincyto mechanism of action to remain effective.
Blincyto Mechanism of Action vs Chemotherapy
The Blincyto mechanism of action differs fundamentally from chemotherapy by offering immune-mediated, targeted cell killing with minimal cross-resistance and superior MRD clearance.
| Aspect | Blincyto Mechanism of Action | Chemotherapy |
|---|---|---|
| Targeting | Immune-directed | Non-specific |
| Cell killing | T-cell mediated | Direct cytotoxic |
| Resistance | Low cross-resistance | High |
| MRD efficacy | High | Limited |
| Immune memory | Possible | None |
Future Directions
The success of the mechanism of action has inspired development of next-generation bispecific antibodies and combination immunotherapy strategies aimed at enhancing efficacy while reducing toxicity.
Conclusion
The Blincyto mechanism of the action represents a revolutionary advancement in leukemia treatment, shifting the therapeutic focus from direct cytotoxicity to immune-mediated precision targeting. By physically linking T-cells to CD19-expressing leukemia cells, Blincyto enables potent, sustained, and selective tumor cell destruction.
Its ability to eradicate minimal residual disease, overcome immune resistance, and induce durable remissions underscores the clinical value of the Blincyto mechanism of the action. While immune-related toxicities require careful management, they are a direct reflection of the therapy’s powerful immunologic activity.
In summary, the Blincyto mechanism action is not just a treatment innovation—it is a cornerstone of modern immuno-oncology that continues to shape the future of targeted cancer therapy.