Table of Contents
Introduction
The treatment landscape for hypertrophic cardiomyopathy (HCM) has dramatically evolved over the past few years. For decades, patients relied primarily on beta-blockers, calcium channel blockers, or invasive procedures like septal myectomy. However, the introduction of cardiac myosin inhibitors revolutionized care. Today, the debate of Aficamten vs Mavacamten is central to discussions among cardiologists, pharmacists, and patients.
When evaluating Aficamten vs Mavacamten, healthcare professionals consider clinical efficacy, safety profile, drug interactions, FDA approval status, monitoring requirements, and patient convenience. Both drugs belong to a ground breaking class of selective cardiac myosin inhibitors, designed to directly target the underlying pathophysiology of obstructive hypertrophic cardiomyopathy (oHCM).
This in-depth guide on will explore mechanism of action, FDA approval, side effects, drug interactions, and head-to-head differences to determine which therapy may offer superior benefits for patients with HCM.
Understanding Hypertrophic Cardiomyopathy (HCM)
Before analyzing , it is essential to understand hypertrophic cardiomyopathy.
HCM is a genetic heart disorder characterized by abnormal thickening of the heart muscle, particularly the left ventricle. This thickening can obstruct blood flow, causing symptoms such as:
- Shortness of breath
- Chest pain
- Fatigue
- Dizziness
- Syncope
- Risk of sudden cardiac death
Obstructive HCM (oHCM) involves left ventricular outflow tract (LVOT) obstruction, which worsens symptoms and reduces cardiac efficiency. Traditional therapies manage symptoms but do not directly address the hypercontractility caused by excessive myosin-actin cross-bridge formation. This is where Aficamten vs Mavacamten becomes highly relevant.
What Is Mavacamten?
Mavacamten is the first FDA-approved cardiac myosin inhibitor for adults with symptomatic obstructive HCM. It works by reducing excessive cardiac contractility through selective inhibition of myosin ATPase activity.
FDA Approval of Mavacamten
Mavacamten received FDA approval in 2022 for the treatment of symptomatic NYHA class II-III obstructive hypertrophic cardiomyopathy in adults. This approval marked a milestone in cardiology.
When discussing Aficamten vs Mavacamten, Mavacamten currently has the advantage of being commercially available and widely studied in real-world settings.
What Is Aficamten?
In the ongoing comparison Aficamten represents the next-generation cardiac myosin inhibitor.
Aficamten is an investigational or recently approved cardiac myosin inhibitor developed to provide:
- Shorter half-life
- More predictable pharmacokinetics
- Reduced need for intensive monitoring
In clinical trials, Aficamten has shown promising results in reducing LVOT gradients and improving symptoms in patients with obstructive HCM.
When evaluating Aficamten vs Mavacamten, Aficamten is often described as a more titratable and flexible alternative.
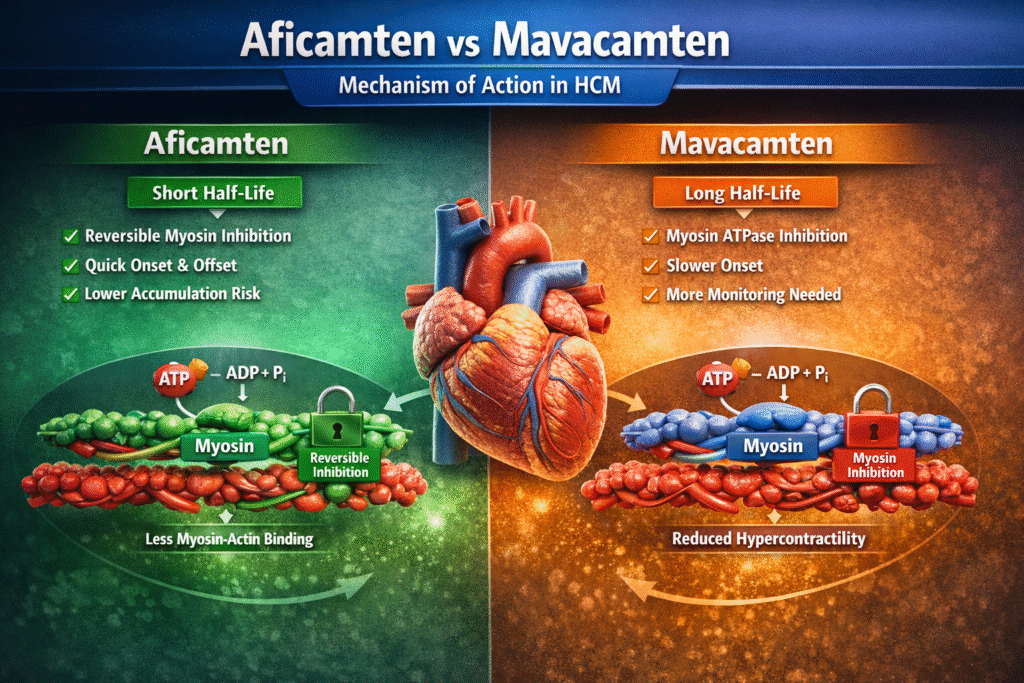
Mavacamten Mechanism of Action
Understanding mechanism of action is crucial .
Mavacamten works by:
- Selectively inhibiting cardiac myosin ATPase
- Reducing actin-myosin cross-bridge formation
- Decreasing hypercontractility
- Lowering LVOT obstruction
- Improving diastolic function
By shifting myosin heads into a super-relaxed state, Mavacamten reduces excessive force generation.
Aficamten Mechanism of Action
In the debate of Aficamten vs Mavacamten, Aficamten shares a similar mechanism but with pharmacologic differences:
- Reversible inhibition of cardiac myosin
- Shorter half-life
- Faster onset and offset
- Reduced accumulation risk
The key difference in Aficamten vs Mavacamten lies not in mechanism category, but in pharmacokinetic behavior and dosing flexibility.
Aficamten vs Mavacamten: Pharmacokinetics Comparison
| Feature | Aficamten | Mavacamten |
| Half-life | Shorter | Longer |
| Steady State | Faster | Slower |
| Dose Adjustment | Easier | Requires monitoring |
| Drug Accumulation | Lower risk | Higher risk |
Because of its shorter half-life, Aficamten may allow more rapid dose adjustments. This is a critical factor in Aficamten vs Mavacamten discussions among clinicians.
Clinical Efficacy:
Clinical trials show both drugs significantly reduce LVOT gradients and improve symptoms.
In comparing Aficamten vs Mavacamten:
- Both improve NYHA functional class
- Both increase exercise capacity
- Both reduce obstruction
- Both improve quality of life scores
However, some data suggest Aficamten may have:
- Lower rates of excessive LVEF reduction
- More predictable response
Still, because Mavacamten has more long-term data, the decision often depends on clinical judgment and patient profile.
Side Effects:
Safety is central in the Aficamten vs Mavacamten comparison.
Common Side Effects of Mavacamten
- Reduced left ventricular ejection fraction (LVEF)
- Heart failure symptoms
- Dizziness
- Syncope
Because of potential LVEF reduction, regular echocardiographic monitoring is required.
Common Side Effects of Aficamten
In trials evaluating Aficamten vs Mavacamten, Aficamten demonstrated:
- Lower incidence of severe LVEF reduction
- Mild dizziness
- Fatigue
- Headache
The shorter half-life may contribute to improved safety flexibility in Aficamten vs Mavacamten discussions.
Drug Interactions
Drug interactions are extremely important in Aficamten vs Mavacamten.
Mavacamten Drug Interactions
Mavacamten is metabolized via CYP2C19 and CYP3A4 pathways.
Avoid with:
- Strong CYP inhibitors
- Strong CYP inducers
- Certain antifungals
- Certain antibiotics
Because of this, medication review is critical in the Aficamten vs Mavacamten decision.
Aficamten Drug Interactions
In the Aficamten vs Mavacamten comparison, Aficamten may have fewer severe interactions due to:
- Shorter half-life
- Potentially simpler metabolism profile
However, drug interaction evaluation remains necessary.
Monitoring Requirements
One of the biggest practical differences in Aficamten vs Mavacamten is monitoring.
Mavacamten requires:
- REMS program enrollment
- Frequent echocardiograms
- Strict LVEF monitoring
Aficamten may require:
- Monitoring
- But potentially less restrictive programs
Thus, in real-world application, Aficamten vs Mavacamten may differ significantly in patient convenience.
Cost and Accessibility
In the economic comparison of Aficamten vs Mavacamten:
- Mavacamten is currently marketed and available
- Aficamten availability depends on regulatory approval status
Cost considerations, insurance coverage, and healthcare system factors influence the Aficamten vs Mavacamten choice.
Which Is Better: Aficamten or Mavacamten?
The answer to Aficamten vs Mavacamten depends on:
- FDA approval status
- Patient risk profile
- Comorbid conditions
- Drug interaction risk
- Access and affordability
Currently, Mavacamten has the advantage of established approval and longer real-world data. However, Aficamten may offer improved dosing flexibility and potentially enhanced safety.
Conclusion
The debate of Aficamten vs Mavacamten represents a new era in precision cardiology. Both drugs directly target the underlying hypercontractility of hypertrophic cardiomyopathy rather than merely treating symptoms.
While Mavacamten is currently the established, FDA-approved therapy, Aficamten shows promise as a next-generation alternative with improved pharmacokinetics and titration flexibility.
Ultimately, the decision of Aficamten vs Mavacamten should be guided by cardiology specialists, patient-specific factors, safety considerations, and evolving clinical data.