Table of Contents
Introduction: Mavacamten FDA Approval
The mavacamten FDA approval represents one of the most significant advancements in cardiovascular medicine in recent years. For decades, patients suffering from hypertrophic cardiomyopathy (HCM) had limited treatment options that primarily focused on symptom relief rather than addressing the root cause. Mavacamten, developed by MyoKardia (acquired by Bristol Myers Squibb), changes this narrative.
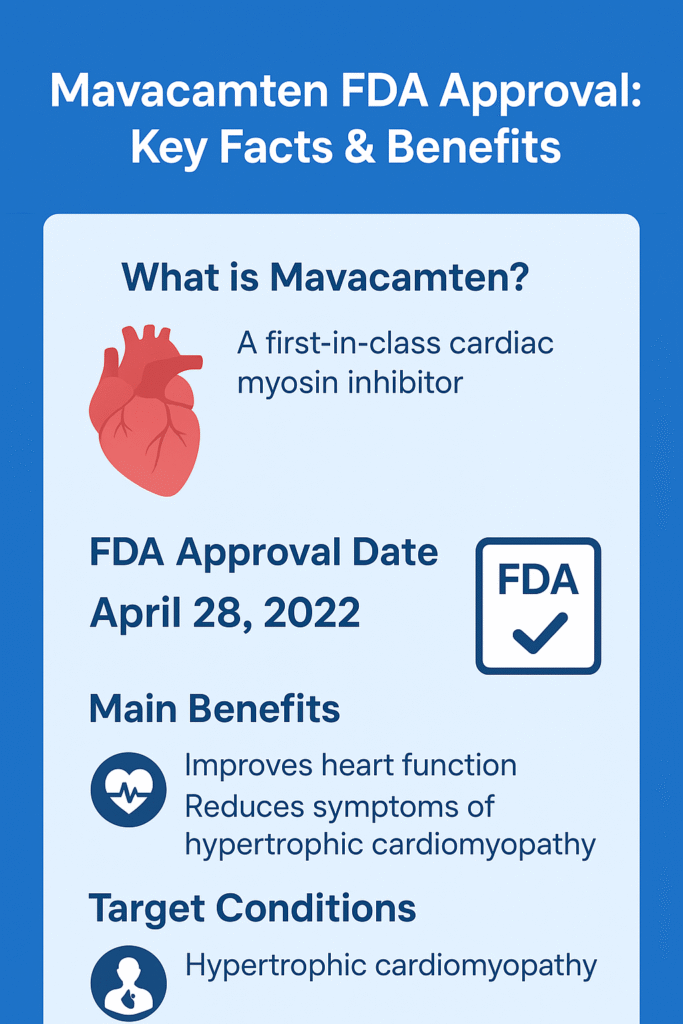
This first-in-class cardiac myosin inhibitor targets the underlying pathophysiology of HCM, offering renewed hope to thousands of patients struggling with this condition. Let’s explore in depth what mavacamten is, how it works, the story behind mavacamten FDA approval , and what it means for patients and clinicians alike.
What Is Mavacamten?
Mavacamten, marketed under the brand name Camzyos, is a novel oral medication designed specifically to treat obstructive hypertrophic cardiomyopathy (oHCM)—a chronic, progressive disease that causes thickening of the heart muscle, particularly the left ventricle.
Unlike older drugs that focus on reducing symptoms like chest pain or shortness of breath, mavacamten acts directly on the molecular machinery of the heart muscle, providing a disease-modifying approach rather than just symptomatic relief.
Understanding Hypertrophic Cardiomyopathy (HCM)
To appreciate the importance of mavacamten FDA approval, it’s crucial to understand the disease it treats.
Hypertrophic cardiomyopathy is a genetic heart condition characterized by abnormal thickening of the heart muscle, particularly the walls of the left ventricle. This thickening can obstruct blood flow from the heart and lead to symptoms such as:
- Shortness of breath (dyspnea)
- Chest pain (angina)
- Palpitations
- Fatigue
- Lightheadedness or fainting
HCM affects approximately 1 in 500 people, making it one of the most common genetic cardiac disorders worldwide. However, due to its subtle symptoms, it often goes undiagnosed until adulthood.
In obstructive HCM, the thickened septum between the heart’s ventricles obstructs blood flow from the left ventricle into the aorta, increasing cardiac workload and reducing oxygen supply to the body. After mavacamten FDA approval , it is on eof the option for Hypertrophic Cardiomyopathy treatment .
Traditional Treatments and Their Limitations
Before mavacamten, treatment for obstructive HCM relied on symptom management through medications such as:
- Beta-blockers (e.g., metoprolol)
- Calcium channel blockers (e.g., verapamil)
- Antiarrhythmics (e.g., disopyramide)
In severe cases, patients underwent invasive procedures like surgical myectomy or alcohol septal ablation to remove or shrink part of the thickened heart muscle.
While effective for some, these approaches did not directly target the molecular cause of the disease—the excessive contractility of cardiac muscle due to overactive myosin heads.
This is where mavacamten stands apart after mavacamten FDA approval.
How Mavacamten Works: Mechanism of Action
Mavacamten’s mechanism of action is what makes it truly revolutionary. It is a selective allosteric inhibitor of cardiac myosin ATPase, the enzyme responsible for generating contractile force in the heart muscle.
In simple terms:
- The drug reduces the number of myosin heads that can interact with actin filaments during heart contraction.
- This leads to decreased hypercontractility of the heart muscle.
- As a result, the left ventricular outflow tract (LVOT) obstruction is reduced.
- The heart functions more efficiently, pumping blood with less stress and improved diastolic filling.
By modulating the sarcomere function, mavacamten directly addresses the underlying cause of hypertrophic cardiomyopathy rather than merely masking the symptoms.
The Road to Mavacamten FDA Approval
The U.S. Food and Drug Administration (FDA) officially approved mavacamten (Camzyos) on April 28, 2022, for adults with symptomatic New York Heart Association (NYHA) Class II-III obstructive hypertrophic cardiomyopathy.
The approval was based primarily on results from pivotal clinical trials, particularly the EXPLORER-HCM and VALOR-HCM studies, which demonstrated significant improvements in symptoms, functional capacity, and quality of life.
Let’s take a closer look at these key studies.
Clinical Trial Highlights
1. EXPLORER-HCM Trial
- Design: Phase 3, double-blind, randomized, placebo-controlled trial
- Participants: 251 patients with symptomatic oHCM
- Duration: 30 weeks
- Results:
- 37% of patients receiving mavacamten achieved the primary endpoint compared with 17% in the placebo group.
- Significant reduction in LVOT gradient and improvement in peak oxygen consumption (VO2).
- Enhanced quality of life measured by Kansas City Cardiomyopathy Questionnaire (KCCQ) scores.
2. VALOR-HCM Trial
- Purpose: Evaluated mavacamten in patients who were eligible for septal reduction therapy (SRT).
- Findings:
- After 16 weeks, 82% of mavacamten-treated patients no longer met SRT criteria versus 23% in the placebo group.
- This demonstrated that the drug could reduce the need for invasive heart surgery in many cases.
3. Long-Term Data
Follow-up studies have shown sustained improvement in LVOT gradient reduction and exercise capacity without significant safety concerns when monitored appropriately.
FDA’s Evaluation and Decision
The FDA’s Cardiovascular and Renal Drugs Advisory Committee reviewed mavacamten’s efficacy and safety profile in detail. Given the consistent, clinically meaningful results and manageable safety profile, the mavacamten FDA approval was granted under standard review.
However, the FDA required that the medication be dispensed under a Risk Evaluation and Mitigation Strategy (REMS) program due to the potential for reduced left ventricular ejection fraction (LVEF)—a known risk of excessive cardiac inhibition.
This ensures patients undergo regular echocardiographic monitoring while on treatment to maintain safety.
Benefits of Mavacamten
1. Disease-Specific Treatment
Mavacamten is the first drug that targets the molecular defect in HCM—hypercontractility caused by excessive myosin-actin cross-bridging.
2. Improved Symptoms and Exercise Capacity
Clinical trials showed a marked improvement in exercise tolerance, symptom relief, and overall cardiac function.
3. Reduced Need for Surgery
Many patients previously considered for septal myectomy or alcohol ablation no longer needed invasive procedures after mavacamten therapy.
4. Enhanced Quality of Life
Patients reported significant improvements in daily functioning, reduced fatigue, and fewer cardiac events.
5. Hope for Non-Obstructive HCM
Ongoing trials are evaluating mavacamten’s role in non-obstructive HCM, potentially expanding its therapeutic reach.
Potential Risks and Side Effects
While mavacamten offers substantial benefits, it is not without risks. The main concern is systolic dysfunction—a reduction in heart’s pumping ability if the dose is too high.
Common Side Effects:
- Dizziness
- Chest discomfort
- Fatigue
- Syncope (fainting)
- Palpitations
Serious Risks:
- Heart failure symptoms due to excessive suppression of cardiac contractility
- Decreased ejection fraction (LVEF <50%), requiring temporary discontinuation
Hence, patients on mavacamten undergo regular echocardiography (every 4–12 weeks) to ensure optimal dosing and prevent complications.
The Significance of Mavacamten FDA Approval
The mavacamten FDA approval is more than a regulatory milestone—it represents a paradigm shift in the treatment of hypertrophic cardiomyopathy.
Here’s why mavacamten FDA approval matters:
- First-in-Class Drug:
Mavacamten is the first approved cardiac myosin inhibitor, introducing a novel therapeutic class in cardiology. - Personalized Medicine:
The drug allows physicians to tailor treatment based on individual patient response and echocardiographic findings. - Reduced Surgical Burden:
It offers a non-invasive alternative to septal reduction surgeries, changing the treatment landscape. - Research Catalyst:
The success of mavacamten has encouraged development of other myosin modulators for heart failure and other cardiac conditions. - Improved Patient Outcomes:
For patients who once faced limited options, mavacamten provides a disease-modifying therapy that improves both survival and quality of life.
Ongoing Studies and Future Outlook
The story doesn’t end with mavacamten FDA approval. Researchers continue to explore its full potential in various cardiac conditions.
1. MAVERICK-HCM Trial
This Phase 2 study investigated mavacamten in non-obstructive HCM. Early results indicated favorable safety and biomarker improvements, paving the way for future FDA submissions for broader indications.
2. Real-World Evidence
Post-marketing studies are assessing long-term effectiveness, patient adherence, and cardiac remodeling effects beyond the clinical trial environment.
3. Combination Therapies
Researchers are exploring how mavacamten might work in combination with beta-blockers or ACE inhibitors to further improve outcomes in complex heart disease cases.
Impact on Patients and the Medical Community
For patients living with obstructive hypertrophic cardiomyopathy, daily activities like walking uphill, climbing stairs, or exercising can be exhausting. Many fear worsening symptoms or sudden cardiac death.
With mavacamten, these individuals now have access to a treatment that can normalize heart function, restore physical capacity, and reduce disease progression.
For cardiologists, it marks the beginning of a new therapeutic era—one where the root cause of heart muscle disease can be modulated at the molecular level.
Hospitals and cardiac care centers have also begun integrating mavacamten treatment protocols into heart failure management programs, improving multidisciplinary care.
Mavacamten in Global Context
Following the mavacamten FDA approval, regulatory agencies across the world—such as the European Medicines Agency (EMA) and Health Canada—have also evaluated the drug for approval.
The EMA granted authorization in 2023 for adults with symptomatic obstructive HCM, making mavacamten a globally recognized therapy.
This rapid international adoption underscores its strong clinical data and transformative potential.
Cost and Accessibility
One major discussion point post-approval is the cost of mavacamten. As a novel targeted therapy, it comes with a premium price tag.
However, Bristol Myers Squibb has implemented patient assistance programs and insurance coverage support to improve accessibility in the U.S. The company also partners with healthcare systems to ensure equitable distribution.
In the long term, as patents expire and competitors develop similar molecules, costs may reduce—making disease-modifying therapy more affordable for all.
Expert Opinions
Leading cardiologists and researchers have hailed mavacamten FDA approval as a landmark achievement.
“For the first time, we have a drug that doesn’t just manage symptoms—it changes the disease itself,” says Dr. Iacopo Olivotto, one of the principal investigators of the EXPLORER-HCM trial.
Medical journals such as The New England Journal of Medicine and Circulation have also published extensive reviews praising mavacamten’s mechanism, safety, and long-term benefits.
Conclusion: The Future of Cardiac Care Has Arrived
The mavacamten FDA approval is more than a scientific milestone—it’s a beacon of hope for millions affected by hypertrophic cardiomyopathy.
By directly targeting the molecular basis of the disease, mavacamten represents a revolutionary shift from symptom management to disease modification. It empowers patients, transforms clinical practice, and opens the door for future innovations in cardiac therapeutics.
As research continues and more real-world data emerge, mavacamten’s legacy will likely extend far beyond HCM, inspiring a new generation of precision medicines for the heart.
Key Takeaways
- Drug Name: Mavacamten (Camzyos)
- Developer: MyoKardia / Bristol Myers Squibb
- Indication: Symptomatic obstructive hypertrophic cardiomyopathy (HCM)
- Mechanism: Cardiac myosin inhibitor reducing hypercontractility
- FDA Approval Date: April 28, 2022
- Benefits: Reduces LVOT obstruction, improves exercise tolerance, and lessens need for surgery
- Risks: Decreased LVEF, heart failure (requires REMS monitoring)