Table of Contents
Introduction: A New Era in Keratoconus Management
Keratoconus is a progressive eye disorder that causes the cornea – the transparent front part of the eye – to thin and take on a cone-like shape. This degeneration causes blurred vision, light sensitivity and, in advanced stages, possible vision loss.
For decades, ophthalmologists have been searching for safer, less invasive and more effective ways to stop the progression of this condition.
Enter Epioxa – a next-generation, incision-free corneal cross-linking (CXL) technology designed to strengthen the cornea without the surgical cuts or discomfort of traditional methods. Epioxa has redefined the standard of keratoconus care by combining clinical precision with patient comfort.
Understanding Keratoconus and the Need for Better Treatments
Keratoconus usually develops in the late teens or early twenties and progresses slowly over time. In this condition, the collagen fibers in the cornea weaken, causing the cornea to lose its natural dome shape. As it bulges further, vision becomes impaired.
Common symptoms of keratoconus include:
• Blurred or distorted vision
• Increased sensitivity to light
• Frequent changes in eyeglass prescription
• Double vision in one eye
• Eye strain and irritation
Eyeglasses and contact lenses can help in the early stages, but they cannot stop the underlying structural weakness. Without treatment, advanced keratoconus may require a corneal transplant.
Traditional corneal cross-linking (CXL) is a major advance, but it often involves removing the corneal epithelium (outer layer), which can cause discomfort, risk of infection, and a long recovery time. This is where Epioxa offers a breakthrough – a truly incision-free alternative with similar or better results.
Epioxa: The Incision-Free Breakthrough
It is a state-of-the-art corneal cross-linking system that strengthens the corneal structure without removing the epithelium. This technique is commonly referred to as “epithelium-on” CXL (epithelium-on), as opposed to the older “epithelium-off” procedures.
By maintaining the integrity of the corneal surface, Epioxa dramatically reduces healing time, discomfort, and the risk of complications while achieving effective stabilization of the corneal shape.
FDA Approval and Clinical Significance
In 2025, the U.S. Food and Drug Administration (FDA) granted approval to Epioxa for the treatment of progressive keratoconus. The approval was based on extensive clinical data demonstrating that Epioxa achieves comparable biomechanical strengthening to traditional CXL, but with significantly fewer side effects.
Highlights of the FDA Approval
- Approval Year: 2025
- Indication: Treatment of progressive keratoconus
- Type: Epithelium-on (incision-free) corneal cross-linking system
- Key Advantage: No epithelial removal or corneal incision required
- Developer: VisionCure Biotechnologies
FDA approval marked a pivotal moment in ophthalmology — finally offering patients a more comfortable, accessible, and effective solution for managing keratoconus.
Mechanism of Action : The Science Behind Corneal Strengthening
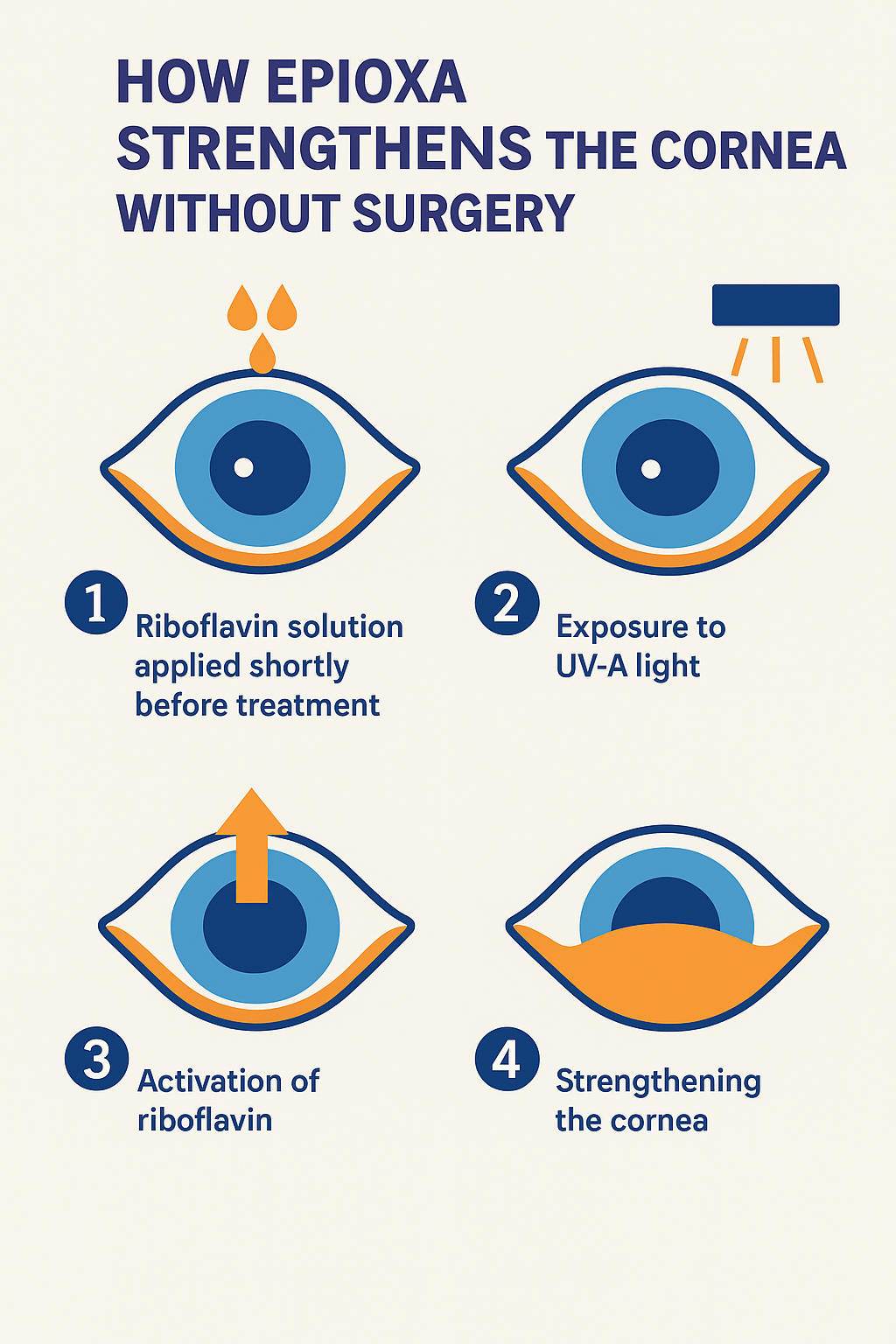
Epioxa’s innovative technology uses a combination of riboflavin (vitamin B2) and controlled ultraviolet-A (UVA) light to increase corneal rigidity. This process strengthens the collagen fibers in the cornea by creating new molecular cross-links between them.
However, what sets Epioxa apart is its unique delivery mechanism that allows riboflavin to penetrate the cornea without removing the epithelium.
Step-by-step process of treatment
1. Preparation: The eye is numbed with anesthetic drops to ensure comfort.
2. Application of Riboflavin: A specially formulated Epioxa Riboflavin solution is applied to the cornea. This solution is enhanced for high epithelial permeability.
3. UVA light exposure: The cornea is exposed to a precise wavelength of UVA light for 10-15 minutes.
4. Cross-link formation: The interaction between riboflavin and UVA light leads to the formation of new collagen cross-links, which strengthens the corneal tissue.
5. Completion: No incision or epithelium removal is required, and the patient can resume normal activities quickly.
This non-invasive method provides the same corneal tightening effects as traditional CXL and provides a more comfortable experience for the patient.
Key Benefits
Epioxa is designed with both efficacy and comfort in mind. It is rapidly becoming the preferred choice for ophthalmologists and patients seeking modern keratoconus care.
1. Incision-Free and Epithelium-On
No removal of the corneal surface means:
- Minimal discomfort
- No open wound
- Lower infection risk
2. Faster Recovery
Patients typically resume normal activities within 24 to 48 hours, compared to up to 7 days with traditional CXL.
3. Reduced Risk of Complications
By preserving the corneal epithelium, it minimizes:
- Infection risk
- Corneal haze formation
- Postoperative pain
4. Proven Long-Term Stability
Clinical trials show that it effectively halts keratoconus progression and improves corneal strength for several years post-treatment.
5. Better Patient Comfort and Vision Quality
Patients report clearer vision and greater satisfaction, thanks to the smooth, non-invasive nature of the procedure.
Clinical Evidence Supporting
Multiple clinical trials conducted globally have demonstrated the safety and efficacy of Epioxa.
Key Clinical Findings
- Corneal Flattening: Average reduction in keratometry (Kmax) by 1.5–2.0 diopters within 12 months.
- Visual Improvement: Significant gain in uncorrected and corrected visual acuity.
- Safety: Over 98% of patients experienced no epithelial damage or infection.
- Patient Satisfaction: 94% reported mild or no discomfort during recovery.
These results position Epioxa as a superior choice for both early and moderate stages of keratoconus.
Comparing Epioxa with Traditional Corneal Cross-Linking
| Parameter | Traditional CXL (Epi-Off) | Epioxa (Epi-On) |
| Corneal epithelium | Removed | Intact |
| Discomfort level | Moderate to severe | Minimal |
| Recovery time | 5–7 days | 1–2 days |
| Risk of infection | Higher | Very low |
| Vision recovery | Delayed | Rapid |
| Patient suitability | Limited (thicker corneas preferred) | Broader eligibility |
The above comparison highlights why Epioxa represents a paradigm shift in the management of keratoconus.
Ideal Candidates for Treatment
Epioxa is suitable for most patients with progressive keratoconus or early signs of corneal weakening.
You may be a good candidate for Epioxa if you:
• Have been diagnosed with progressive keratoconus
• Are experiencing increasing astigmatism or blurred vision
• Have a corneal thickness of at least 400 microns
• Want to avoid surgical incisions or a long recovery period
However, patients with severe corneal scarring, advanced keratoconus, or autoimmune disease should consult an ophthalmologist for an individualized evaluation.
Procedure for Treatment
Epioxa is performed in an outpatient clinical setting and typically takes 30–40 minutes per eye.
Step-by-Step Experience:
- Eye drops are applied to numb the cornea.
- Riboflavin drops are administered for 10–15 minutes.
- UVA light exposure follows for another 10 minutes.
- Post-procedure eye drops are given to enhance healing.
- Patients can go home the same day — no hospital stay required.
Post-Treatment Recovery and Care
One of the biggest benefits of Epixoxa is its rapid recovery.
Aftercare guidelines include:
• Use antibiotic and lubricating eye drops as prescribed by your doctor for 5-7 days.
• Avoid rubbing your eyes for a week.
• Wear sunglasses to protect from UV rays.
• Attend follow-up appointments as recommended.
Patients usually experience clear vision within a few days and continue to improve over the next few months.
Potential Side Effects
Epioxa is extremely safe, but like all medical procedures, minor temporary effects can occur:
- Mild dryness or irritation
- Slight light sensitivity
- Transient blurred vision
These symptoms usually resolve within a few days without intervention.
Epioxa vs. Corneal Transplant: The Non-Surgical Advantage
In advanced keratoconus, corneal transplantation (keratoplasty) was once the only solution. Epioxa changes that by stabilizing the cornea early, often preventing the need for transplants altogether.
Advantages Over Transplant Surgery:
- No surgical incision
- No graft rejection risk
- Faster vision recovery
- Cost-effective and outpatient-friendly
This makes Epioxa a preferred early intervention for patients wishing to preserve their natural cornea.
Cost and Accessibility
The cost can vary by clinic and location, but Epioxa is generally more affordable than surgical procedures. Insurance coverage is increasing, as Epioxa is now an FDA-approved treatment for keratoconus. Many clinics also offer flexible financing options to make the procedure accessible to a wider range of patients.
The Future of Corneal Cross-Linking
Epioxa represents not only a technological advance but also a change in the philosophy of corneal care. Researchers are already exploring its use in other corneal conditions, such as post-LASIK ectasia and corneal thinning disorders. Future generations of Epioxa technology may further reduce treatment times and enhance biomechanical outcomes – continuing to improve the safety and comfort of corneal strengthening.
Conclusion
Epioxa has changed the landscape of keratoconus treatment. Its incision-free, epithelium-on approach offers patients a safer, more comfortable, and equally effective alternative to traditional corneal cross-linking.
By preserving the natural integrity of the cornea while strengthening its structure, it allows individuals to maintain their vision, confidence, and quality of life without surgery.
As the next frontier in ophthalmology, Epioxa stands as a testament to modern innovation: combining science, safety, and patient comfort for a brighter tomorrow.
FAQs

