Table of Contents
Introduction
Bladder cancer remains one of the most prevalent and recurring cancers in the world. While early-stage disease can often be treated successfully, certain patients face persistent recurrence despite conventional therapy. For adults with non-muscle invasive bladder cancer (NMIBC) that does not respond to Bacillus Calmette–Guérin (BCG), new hope has arrived in the form of Inlexzo — a novel, targeted therapy designed to preserve the bladder and improve treatment outcomes.
This article explores everything about Inlexzo — what it is, how it works, its benefits, potential side effects, and where it fits in the current treatment landscape.
Understanding NMIBC and the BCG Challenge
What Is NMIBC?
Non-muscle invasive bladder cancer (NMIBC) refers to cancer that grows within the inner lining of the bladder but hasn’t invaded the muscle wall. These cancers are typically classified into stages Ta, T1, and carcinoma in situ (CIS). Although NMIBC is less aggressive than muscle-invasive forms, it has a high recurrence rate, requiring vigilant monitoring and repeated treatments.
Role and Limitations of BCG Therapy
For decades, BCG therapy has been the standard for NMIBC. It involves instilling a weakened strain of bacteria into the bladder to stimulate the immune system to attack cancer cells. However, about 30–40% of patients either do not respond to BCG or experience recurrence after initial success. These patients are termed BCG-unresponsive.
Historically, the only curative option for BCG-unresponsive NMIBC was radical cystectomy — surgical removal of the bladder — which carries significant risks and impacts quality of life. This unmet need for bladder-preserving therapy paved the way for Inlexzo.
What Is Inlexzo?
Inlexzo (gemcitabine intravesical system) is a novel drug-device combination that provides continuous and targeted delivery of the chemotherapy drug gemcitabine directly to the bladder wall.
Unlike standard intravesical therapy (where a drug is retained for 1–2 hours), Inlexzo’s special design allows gemcitabine to be released slowly over several weeks — maintaining constant exposure to cancer cells while minimizing systemic absorption.
This approach enhances treatment effectiveness and reduces side effects typically associated with intravenous chemotherapy.
How Inlexzo Works ?
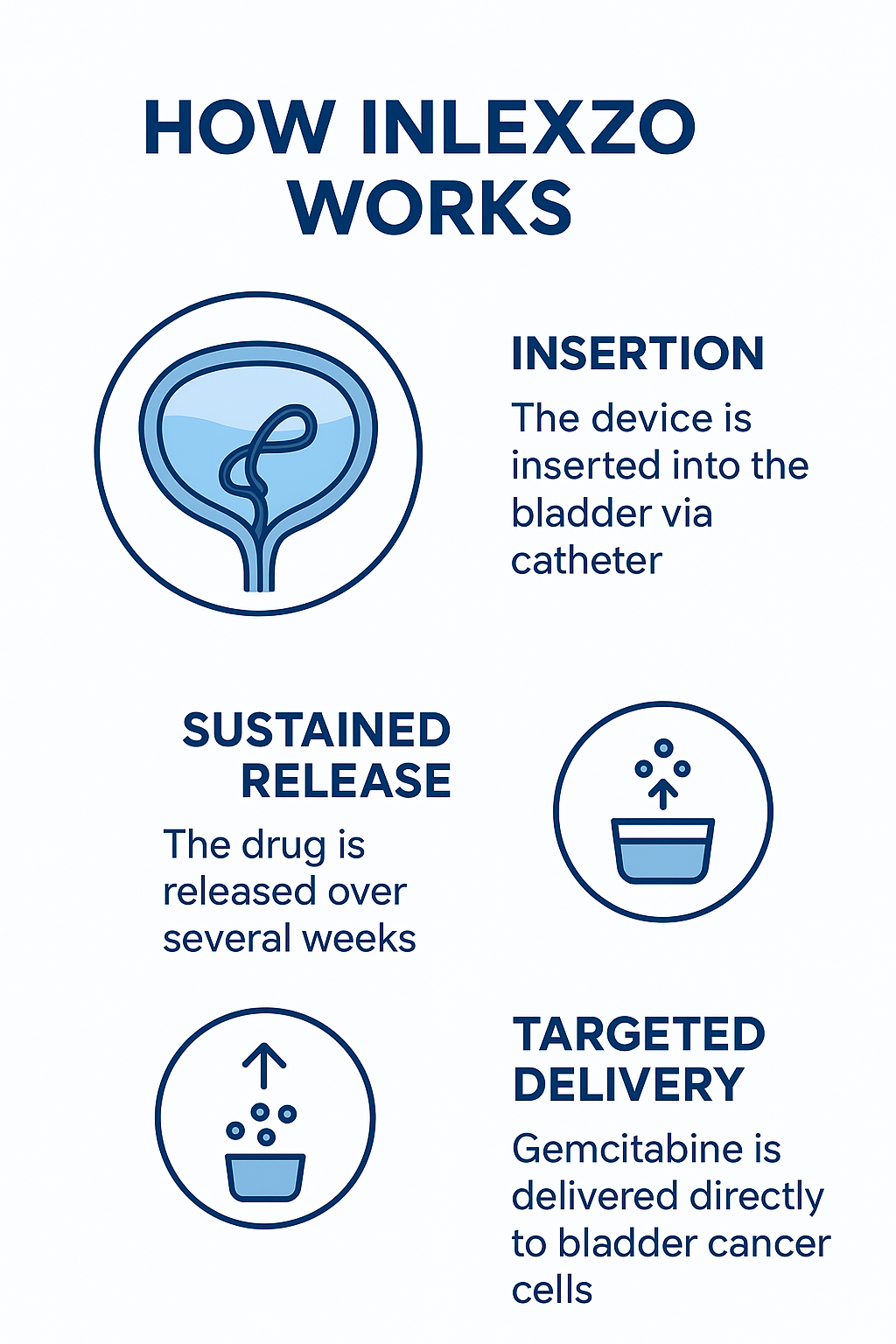
Inlexzo utilizes an intravesical drug delivery system, often described as a flexible, coiled device inserted into the bladder through a catheter. Once placed, it remains inside the bladder for about three weeks, continuously releasing gemcitabine.
Here’s how it functions step by step:
- Insertion – The device is inserted into the bladder via a catheter in an outpatient procedure.
- Sustained Release – Gemcitabine is released gradually into the urine, maintaining therapeutic levels in contact with the bladder lining.
- Removal and Replacement – After each three-week period, the device is removed and replaced according to the treatment schedule.
This steady release creates a longer therapeutic window compared to conventional therapies, allowing enhanced cancer cell exposure while limiting systemic toxicity.
Clinical Evidence Supporting gemcitabine intravesical system
The SunRISe-1 Trial
The pivotal clinical trial evaluating Inlexzo was conducted in adults with BCG-unresponsive NMIBC. In this study, patients with carcinoma in situ (CIS) — with or without papillary tumors — received Inlexzo every three weeks for six months, followed by maintenance cycles every 12 weeks.
Key findings included:
- Complete response rate: Approximately 82% of patients achieved complete remission, showing no detectable disease after treatment.
- Durability: Around 51% of those responders maintained their remission for at least 12 months.
- Safety profile: The therapy was generally well tolerated, with most side effects localized to the urinary tract.
These results marked a major milestone for bladder cancer patients who previously had limited therapeutic options.
Mechanism of Action: How Gemcitabine in Inlexzo Works
Gemcitabine is a nucleoside analog that interferes with DNA synthesis, ultimately leading to cancer cell death. When delivered via Inlexzo, its effectiveness is amplified by:
- Continuous Exposure – Maintaining a steady drug concentration prevents cancer cell regrowth between treatments.
- Localized Therapy – Drug release is confined to the bladder, minimizing systemic exposure and reducing side effects.
- Enhanced Contact Time – The device’s extended presence ensures consistent interaction with the bladder lining, improving drug absorption.
This sustained and localized approach differentiates Inlexzo from traditional intravesical therapies.
Who Can Receive Inlexzo?
Inlexzo is approved for adult patients with:
- Non-muscle invasive bladder cancer (NMIBC)
- Carcinoma in situ (CIS), with or without papillary tumors
- Disease that has not responded to BCG therapy
- No evidence of cancer spread beyond the bladder
It is particularly suitable for individuals who wish to avoid or are medically unfit for radical cystectomy.
Contraindications
Inlexzo should not be used in:
- Patients with bladder perforation or compromised bladder walls
- Those allergic to gemcitabine or device components
- Pregnant women (due to potential fetal toxicity)
Close monitoring by a urologic oncologist is necessary during treatment.
Administration & Dosing Schedule
The dosing regimen generally includes:
- Induction Phase: Device inserted every three weeks for six months (total of 8 cycles).
- Maintenance Phase: After initial response, reinsert every 12 weeks for up to 18 months.
- Each Device Contains: Approximately 225 mg of gemcitabine released continuously.
- Duration in Bladder: Roughly 21 days per cycle.
The procedure is usually performed under local anesthesia in a clinical setting and does not require hospitalization.
Benefits of Inlexzo
1. High Response Rate
Clinical studies have shown inlexzo achieves high complete response rates in patients who previously had limited options.
2. Durable Outcomes
Many patients maintain long-term remission, reducing recurrence risk and delaying disease progression.
3. Bladder Preservation
Unlike radical cystectomy, Inlexzo offers an organ-preserving alternative, helping maintain quality of life.
4. Minimal Systemic Side Effects
Because the drug stays within the bladder, systemic toxicity such as hair loss or nausea is rare.
5. Convenient Outpatient Therapy
The insertion and removal procedures are straightforward and can be performed without general anesthesia.
Potential Side Effects and Risks
Common Adverse Effects
Most side effects are related to urinary irritation and include:
- Frequent urination
- Urinary tract infection
- Painful urination (dysuria)
- Blood in urine (hematuria)
- Bladder discomfort or burning sensation
These effects are generally mild to moderate and resolve after device removal.
Serious Reactions (Less Common)
- Bladder perforation or infection
- Severe inflammation
- Laboratory abnormalities (temporary changes in liver or kidney tests)
- Hypersensitivity or allergic reactions
Monitoring by healthcare professionals throughout treatment helps manage these risks effectively.
Key Warnings and Precautions
- Bladder Integrity: Avoid use in patients with bladder perforation.
- Delayed Surgery Risk: For non-responders, delaying cystectomy could allow disease progression.
- MRI Precautions: Device safety during MRI scans should be verified before imaging.
- Pregnancy Risk: Gemcitabine can cause fetal harm; contraception is advised for both men and women during and after therapy.
Limitations and Challenges
Despite its promise, Inlexzo is not without limitations:
- Lack of Randomized Comparison: Most data are from single-arm studies without control groups.
- Limited Long-Term Data: Durability beyond two years is still being evaluated.
- Potential Device Issues: Risk of device migration, infection, or mechanical complications.
- Cost and Accessibility: Advanced intravesical systems may not be readily available in all regions.
- Strict Monitoring Required: Frequent cystoscopy and follow-up are necessary to detect recurrence early.
Nevertheless, its benefits as a bladder-sparing alternative are substantial.
Inlexzo in the Current Treatment Landscape
Inlexzo represents a new class of bladder-preserving therapy that bridges the gap between standard intravesical chemotherapy and radical cystectomy.
Comparison with Other Options
- BCG Therapy: Inlexzo is used when BCG fails.
- Systemic Immunotherapy: Drugs like checkpoint inhibitors target immune mechanisms but may cause systemic side effects.
- Intravesical Chemotherapy: Traditional short-duration instillations offer less sustained exposure than Inlexzo.
- Radical Cystectomy: Definitive but invasive; Inlexzo provides an alternative for eligible patients.
Patient Journey: What to Expect
- Pre-Treatment Evaluation: Cystoscopy, urine tests, and imaging confirm eligibility.
- Insertion Procedure: Outpatient catheter placement, taking only a few minutes.
- Treatment Cycle: Device remains for three weeks, releasing gemcitabine continuously.
- Follow-Up: Regular cystoscopy and urine cytology to monitor response.
- Maintenance Therapy: Continued treatment every 12 weeks if effective and tolerated.
This structured approach helps achieve sustained response and early detection of recurrence.
Future Directions for Inlexzo
Research is ongoing to explore:
- Combination Therapies: Pairing Inlexzo with immunotherapies for improved outcomes.
- Biomarker Studies: Identifying which patients respond best.
- Long-Term Data: Assessing survival, progression, and bladder preservation rates.
- Cost-Effectiveness: Evaluating accessibility and reimbursement across healthcare systems.
As data accumulate, Inlexzo’s role in NMIBC management will continue to evolve.
Conclusion
Inlexzo marks a major advancement in the treatment of non-muscle invasive bladder cancer (NMIBC) that is unresponsive to BCG. By combining gemcitabine with an innovative, sustained-release intravesical delivery system, it offers high response rates, improved tolerability, and the potential for bladder preservation.
While long-term outcomes and broader clinical experience are still unfolding, Inlexzo has already changed the therapeutic landscape for NMIBC, giving new hope to patients who previously faced limited choices.