Table of Contents
Introduction
On July 2, 2025, the U.S. Food and Drug Administration (FDA) granted accelerated approval to linvoseltamab-gcpt (brand name: Lynozyfic) for the treatment of adults with relapsed or refractory multiple myeloma (R/R MM) who have received at least four prior lines of therapy.
This linvoseltamab FDA approval represents a major milestone in oncology, especially for patients with limited therapeutic options. Multiple myeloma is a challenging blood cancer, and those with relapsed or refractory disease often face poor outcomes. With linvoseltamab, a bispecific antibody therapy, hope is renewed for patients who had exhausted other treatments.
In this article, we will explore:
- The details of the linvoseltamab FDA approval
- How linvoseltamab works
- Clinical trial results supporting approval
- Benefits and risks
- Dosage and administration
- Comparisons with other therapies
- Future research directions
- FAQs for patients and caregivers
What is Linvoseltamab?
Linvoseltamab (Lynozyfic) is a bispecific T-cell engager antibody that targets BCMA (B-cell maturation antigen) on multiple myeloma cells and CD3 on T cells. By binding simultaneously, it redirects the patient’s immune system to attack and kill malignant plasma cells.
Why BCMA?
BCMA is highly expressed in multiple myeloma cells, making it a prime therapeutic target. Several other drugs like teclistamab and elranatamab also target BCMA, but linvoseltamab offers unique binding properties and a different dosing schedule, potentially improving patient outcomes.
Linvoseltamab FDA Approval Details
- Approval Date: July 2, 2025
- Brand Name: Lynozyfic
- Generic Name: Linvoseltamab-gcpt
- Indication: Treatment of adult patients with relapsed or refractory multiple myeloma after ≥4 prior lines of therapy.
- Approval Pathway: Accelerated Approval (based on clinical trial response rates).
- Manufacturer: Regeneron Pharmaceuticals.
This linvoseltamab FDA approval was based on promising clinical trial data, particularly from the LINKER-MM1 trial, which demonstrated meaningful response rates in heavily pretreated patients.
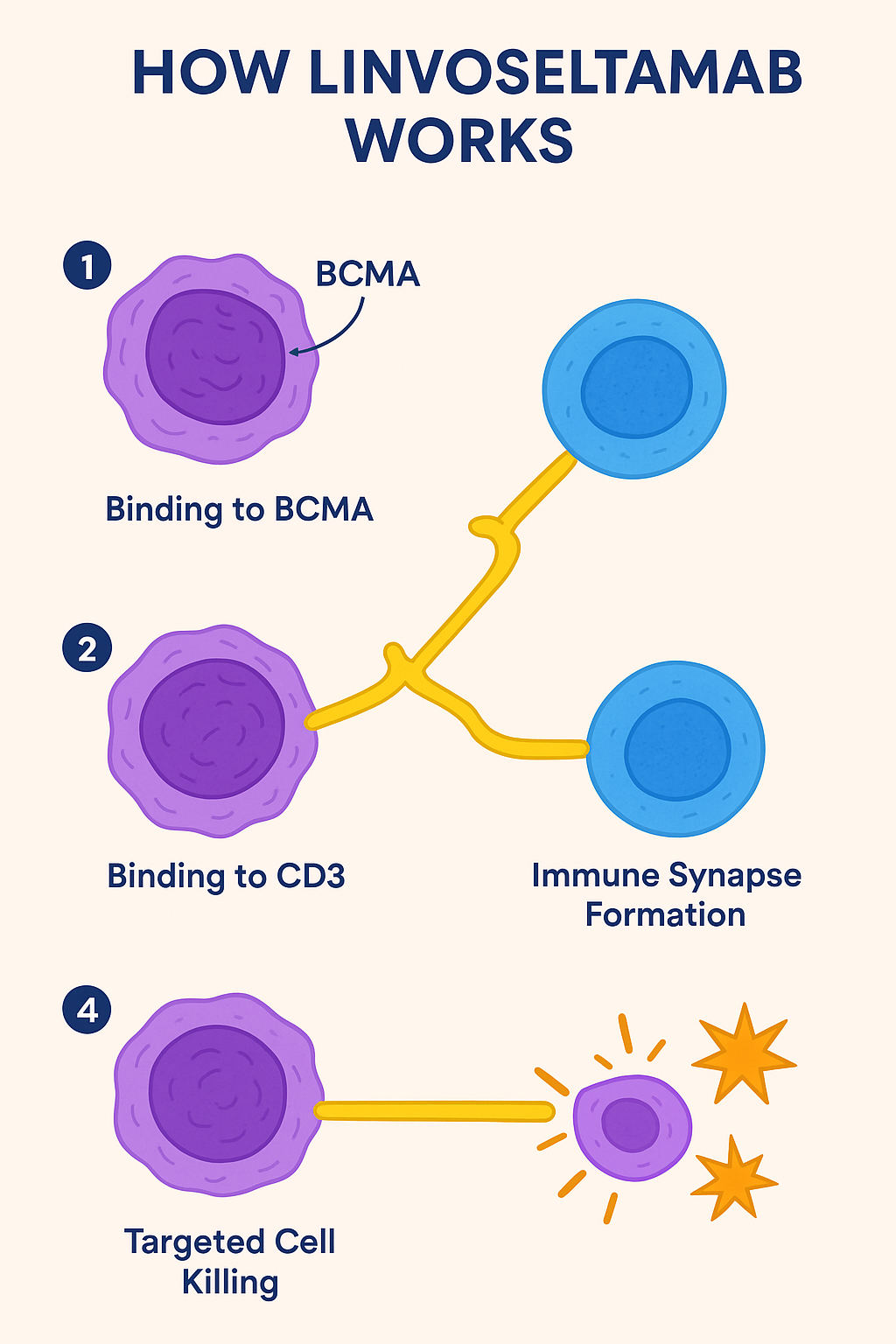
How Does Linvoseltamab Work?
Linvoseltamab belongs to the class of bispecific antibodies. Its mechanism of action involves:
- Binding to BCMA: Located on malignant plasma cells.
- Binding to CD3: Found on T cells, activating them.
- Immune Synapse Formation: Brings T cells into close proximity with cancer cells.
- Targeted Cell Killing: Activated T cells release cytotoxic granules, inducing cancer cell death.
This immune-mediated approach is precision-based, minimizing damage to normal cells and enhancing anti-myeloma activity.
Clinical Trial Evidence Supporting FDA Approval
The linvoseltamab FDA approval was primarily supported by the LINKER-MM1 Phase 2 study.
Key Highlights:
- Patient Population: Adults with relapsed or refractory multiple myeloma who had received ≥4 prior therapies.
- Overall Response Rate (ORR): Nearly 70% in evaluable patients.
- Complete Response (CR) or Better: Around 30%, even in heavily pretreated patients.
- Median Duration of Response (DoR): Over 9 months, with ongoing responses at data cutoff.
- Safety Profile: Manageable side effects with optimized step-up dosing.
These results demonstrated that linvoseltamab is a highly effective and durable therapy, leading to FDA’s accelerated approval.
Benefits of Linvoseltamab (Lynozyfic)
Patients and physicians are excited about the linvoseltamab FDA approval because of its potential benefits:
- New Hope for Difficult-to-Treat Patients: Effective for patients with multiple relapses.
- Rapid Onset of Action: Clinical responses were observed within weeks.
- Durability of Response: Long-lasting remissions in responders.
- Convenient Dosing: Step-up dosing reduces cytokine release syndrome risk.
- Immunotherapy Option: Harnesses patient’s own immune system.
Risks and Side Effects
Like all cancer therapies, linvoseltamab carries potential risks.
Common Side Effects:
- Cytokine Release Syndrome (CRS)
- Fatigue
- Fever
- Low blood counts (neutropenia, anemia, thrombocytopenia)
- Infections
Serious Adverse Events:
- Severe infections (including pneumonia, sepsis)
- Neurological events (confusion, headache, dizziness)
- Infusion-related reactions
Mitigation strategies such as premedication and monitoring protocols are implemented to reduce risks.
Dosage and Administration
Linvoseltamab is administered as an intravenous infusion in specialized healthcare settings.
- Step-Up Dosing Schedule: Gradually increasing doses over initial cycles to lower CRS risk.
- Maintenance Dosing: Administered every few weeks after initial cycles.
- Premedication Required: Corticosteroids, antihistamines, and antipyretics to prevent infusion reactions.
The exact regimen may be tailored based on patient tolerance and disease progression.
Comparison with Other Multiple Myeloma Treatments
The linvoseltamab FDA approval places it alongside other BCMA-targeting therapies.
| Therapy | Type | FDA Approval Year | Key Features |
| Teclistamab | Bispecific antibody | 2022 | First-in-class BCMA/CD3 bispecific |
| Elranatamab | Bispecific antibody | 2023 | Similar mechanism, different dosing |
| CAR-T (ide-cel, cilta-cel) | Cell therapy | 2021+ | Personalized, highly effective, but logistically complex |
| Linvoseltamab (Lynozyfic) | Bispecific antibody | 2025 | Convenient dosing, promising durability, accessible therapy |
Linvoseltamab offers a balance between efficacy and accessibility, unlike CAR-T therapies that require complex manufacturing.
Future of Linvoseltamab Research
The linvoseltamab FDA approval is just the beginning. Ongoing studies aim to:
- Evaluate earlier-line use in multiple myeloma.
- Combine linvoseltamab with other agents (e.g., lenalidomide, daratumumab).
- Test its effectiveness in different myeloma subgroups.
- Explore potential use in other BCMA-positive cancers.
These studies may eventually lead to full FDA approval and expanded indications.
Impact on Patients and the Healthcare System
The linvoseltamab FDA approval has far-reaching implications:
- For Patients: Provides a lifeline therapy after exhausting prior treatments.
- For Caregivers: Offers new hope and extended survival for loved ones.
- For Oncologists: Expands the treatment arsenal in relapsed/refractory multiple myeloma.
- For Healthcare Systems: May improve overall outcomes but raises considerations about cost and accessibility.
Conclusion
The linvoseltamab FDA approval marks a new era in the management of relapsed or refractory multiple myeloma. As an innovative bispecific antibody therapy, it offers patients a much-needed option when other treatments fail.
With ongoing studies, the future looks promising for linvoseltamab. For patients and families battling multiple myeloma, this therapy represents not only scientific progress but also a renewed sense of hope.

