Table of Contents
The rilzabrutinib FDA approval on August 29, 2025, introduced a groundbreaking therapy for patients struggling with immune thrombocytopenia (ITP) and other immune-mediated conditions. Marketed as Wayrilz, rilzabrutinib is a selective oral Bruton’s tyrosine kinase (BTK) inhibitor that brings new hope to patients who often face limited treatment options and chronic complications.
This milestone approval is significant not only for those living with ITP but also for the broader landscape of autoimmune disease management. In this comprehensive article, we will explore what rilzabrutinib is, how it works, the details of its FDA approval, its clinical trial data, benefits, side effects, and what this approval means for the future of immune disorder treatments.
What is Rilzabrutinib?
Rilzabrutinib is an innovative oral therapy designed to selectively inhibit Bruton’s tyrosine kinase (BTK), a key enzyme that plays a role in the signaling pathways of immune cells such as B cells and myeloid cells. By targeting BTK, rilzabrutinib helps regulate overactive immune responses, making it valuable for treating diseases caused by immune dysregulation.
Marketed under the brand name Verilz, rilzabrutinib is specifically approved for the treatment of immune thrombocytopenia (ITP), a rare but serious autoimmune disorder in which the body attacks and destroys its own platelets, leading to abnormal bleeding and bruising.
Rilzabrutinib FDA Approval – Key Details
- Drug name: Rilzabrutinib
- Brand name: Wayrilz
- Approval date: August 29, 2025
- Indication: Treatment of immune thrombocytopenia (ITP) in adults
- Drug class: Bruton’s tyrosine kinase (BTK) inhibitor
- Administration: Oral
The rilzabrutinib FDA approval was based on strong evidence from multiple clinical trials, showing significant improvement in platelet counts, reduced bleeding risk, and overall quality of life in patients with chronic ITP.
Understanding Immune Thrombocytopenia (ITP)
To understand the importance of rilzabrutinib, let’s first look at the disease it treats.
Immune thrombocytopenia (ITP) is an autoimmune disorder where the immune system mistakenly attacks platelets. Without enough platelets, the blood cannot clot normally, leading to:
• Excessive bruising
• Nosebleeds
• Bleeding from the gums
• Heavy menstrual bleeding
• Life-threatening internal bleeding in severe cases
Traditional treatments include corticosteroids, intravenous immunoglobulin (IVIG), and in some cases, splenectomy. However, these options often have limited long-term effectiveness and have significant side effects.
This is where the rilzabrutinib FDA approval becomes so important, providing patients with a safer and more effective option.
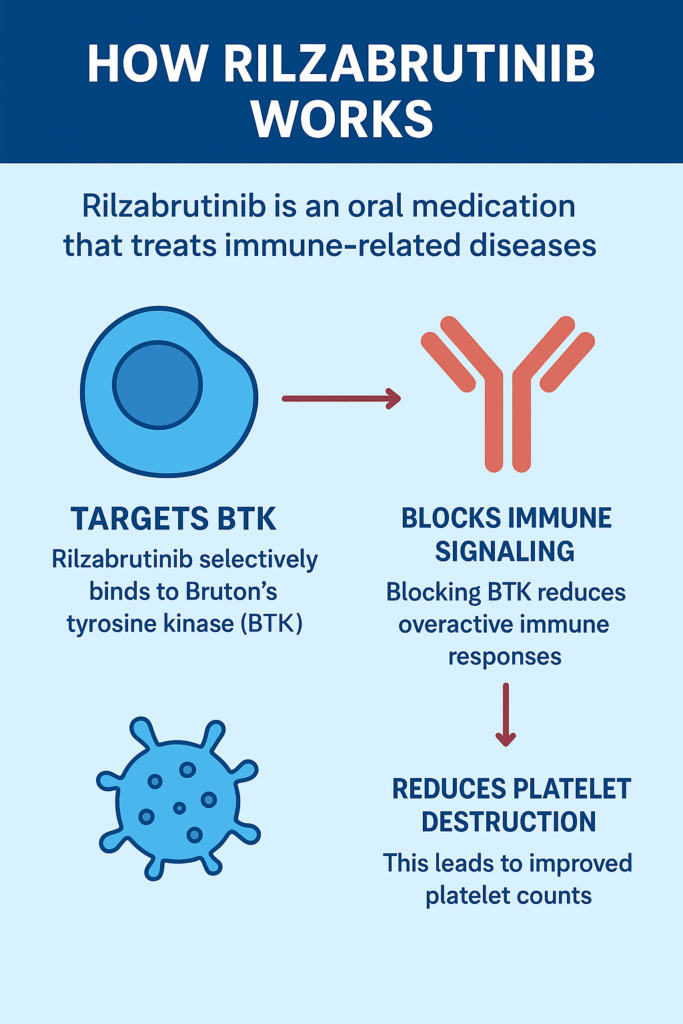
Mechanism of Action: How Rilzabrutinib Works
Rilzabrutinib works by irreversibly binding to Bruton’s tyrosine kinase (BTK).
1. Role of BTK in the immune system: BTK is essential in signaling pathways of B cells and other immune cells that contribute to autoimmune reactions.
2. Blocking BTK: By inhibiting BTK, rilzabrutinib reduces abnormal immune activity without significantly suppressing the overall immune system.
3. Effects: It reduces platelet destruction, improves platelet counts, and reduces bleeding in patients with ITP.
Unlike traditional immunosuppressants, rilzabrutinib’s targeted action makes it effective and relatively safe, as it does not compromise overall immune defense to the same extent.
Clinical Trials Behind Rilzabrutinib FDA Approval
The FDA’s decision was supported by robust clinical trial data:
- Study design: Patients with chronic immune thrombocytopenia who had inadequate responses to standard therapies were enrolled.
- Primary endpoint: Achievement of durable platelet response.
- Results:
- A significant percentage of patients achieved sustained platelet counts above the threshold considered protective against bleeding.
- Many patients reported improved quality of life and fewer bleeding episodes.
- The safety profile was manageable, with mostly mild-to-moderate side effects.
These results demonstrated that rilzabrutinib could provide long-term stability for patients living with ITP, leading directly to rilzabrutinib FDA approval in 2025.
Benefits of Rilzabrutinib (Wayrilz)
The rilzabrutinib FDA approval represents several key benefits for patients:
- Oral administration: No need for injections or hospital visits.
- Targeted mechanism: Reduces immune overactivation without complete suppression.
- Improved platelet counts: Lowers bleeding risk.
- Durable response: Sustained benefits observed in clinical trials.
- Better quality of life: Less fatigue, fewer hospitalizations, and improved day-to-day functioning.
Potential Side Effects of Rilzabrutinib
Like all medications, rilzabrutinib is not without side effects. Common side effects observed during trials included:
- Headache
- Diarrhea
- Nausea
- Upper respiratory tract infections
- Fatigue
More serious but less frequent side effects may include increased risk of infection and liver enzyme abnormalities.
Patients should discuss risks and benefits with their healthcare providers before starting treatment.
Comparison With Other ITP Treatments
| Treatment | Administration | Limitations | How Rilzabrutinib Helps |
| Corticosteroids | Oral/IV | Long-term side effects, relapse common | Offers long-term benefit with targeted action |
| IVIG | IV infusion | Short-lived effect, costly | Oral alternative, durable results |
| Splenectomy | Surgery | Invasive, permanent, not always effective | Non-surgical treatment |
| Thrombopoietin receptor agonists (TPO-RAs) | Oral/Injectable | May cause rebound effect | Provides alternative mechanism |
Clearly, rilzabrutinib provides a much-needed new option in the treatment landscape.
Market Impact of Rilzabrutinib FDA Approval
The rilzabrutinib FDA approval is expected to have significant market implications:
- For patients: A new standard of care for ITP.
- For physicians: An effective oral treatment to reduce dependency on steroids and IVIG.
- For the pharmaceutical industry: Reinforces the importance of BTK inhibitors in autoimmune diseases.
The approval also paves the way for studying rilzabrutinib in other immune-mediated disorders, such as pemphigus vulgaris, multiple sclerosis, and lupus.
Future Outlook: Beyond ITP
While the rilzabrutinib FDA approval currently covers ITP, ongoing research is exploring its potential in a wide range of autoimmune conditions. Clinical trials are underway for:
- Pemphigus vulgaris (a blistering skin disease)
- Multiple sclerosis
- Rheumatoid arthritis
- Other B-cell mediated autoimmune disorders
This means rilzabrutinib may soon become a cornerstone therapy across multiple immune-related diseases.
Conclusion
The FDA approval of rilzabrutinib is a historic step forward in the management of immune thrombocytopenia (ITP) and potentially other autoimmune conditions. Marketed as Verilz, this oral BTK inhibitor offers patients a safer, more effective, and more convenient alternative to traditional treatments.
By specifically targeting BTK, rilzabrutinib reduces platelet destruction, reduces the risk of bleeding, and improves quality of life. Its approval is not only a win for patients with ITP but also a promising sign for the future of autoimmune disease treatment.
As clinical research expands, rilzabrutinib could emerge as a transformative therapy in many immune-mediated diseases, solidifying its place in modern medicine.
Frequently Asked Questions (FAQs)

1 thought on “Rilzabrutinib FDA Approval: A Game-Changer for Immune Thrombocytopenia and Beyond”