Table of Contents
Vision loss is one of the biggest life-changing problems for any individual. In March 2025, the approval of the Encelto implant brought new hope to patients with idiopathic macular telangiectasia type 2 (MacTel type 2). This allogeneic encapsulated cell-based gene therapy is designed to preserve vision in adults at risk of progressive vision loss due to MacTel.
In this article, we will explore everything you need to know about the Encelto implant, including its mechanism of action, benefits, surgical procedure, side effects, eligibility criteria, and its future for ophthalmology.
What is Encelto Implant?
Encelto (scientific name: revakinagenetaroretsel-lwey) is a surgically implanted A device developed as a novel therapy for patients with MacTel Type 2, a rare retinal disease.
• It is an encapsulated cell-based gene therapy that delivers therapeutic proteins directly into the eye.
• The therapy is designed to slow the progression of the disease and preserve vision in adults.
• The US Food and Drug Administration (FDA) approved Encelto on March 6, 2025, making it the first cell-based gene therapy for MacTel.
This advancement provides an alternative option for patients where standard treatment options are limited or non-existent.
Understanding MacTel Type 2
Idiopathic macular telangiectasia type 2 is a rare, progressive eye disorder that affects the macula, the part of the retina responsible for central vision.
• It is characterized by abnormal blood vessels and degeneration of macular tissue.
• Patients often experience blurred central vision, difficulty reading, and distorted images.
• Over time, the condition can lead to irreversible vision loss.
Since no curative treatment previously existed for MacTel type 2, the arrival of the Encelto implant is a revolutionary advance.
How Does Encelto Implant Work?
The mechanism of action is unique and highly innovative.
- Encapsulation Technology
- The implant contains genetically modified allogeneic cells encapsulated within a semi-permeable membrane.
- These cells continuously release therapeutic proteins into the retina.
- Sustained Delivery
- The device ensures long-term delivery of therapeutic molecules, eliminating the need for frequent injections.
- Vision Preservation
- The released proteins slow the degeneration of retinal cells, helping to preserve functional vision in patients with MacTel Type 2.
This cell-based sustained delivery system makes Encelto a paradigm shift in ocular gene therapy.
FDA Approval of Encelto Implant
The FDA approval of Encelto on March 6, 2025, was a landmark moment in ophthalmology.
• The approval came after Encelto was shown to significantly slow the progression of MacTel type 2 in multiple Phase 3 clinical trials.
• Patients reported improved vision stability compared to patients treated with placebo.
• The approval underscores the potential of gene therapy and regenerative medicine to treat previously untreatable retinal disorders.
Benefits
The Encelto implant has several advantages over traditional treatments:
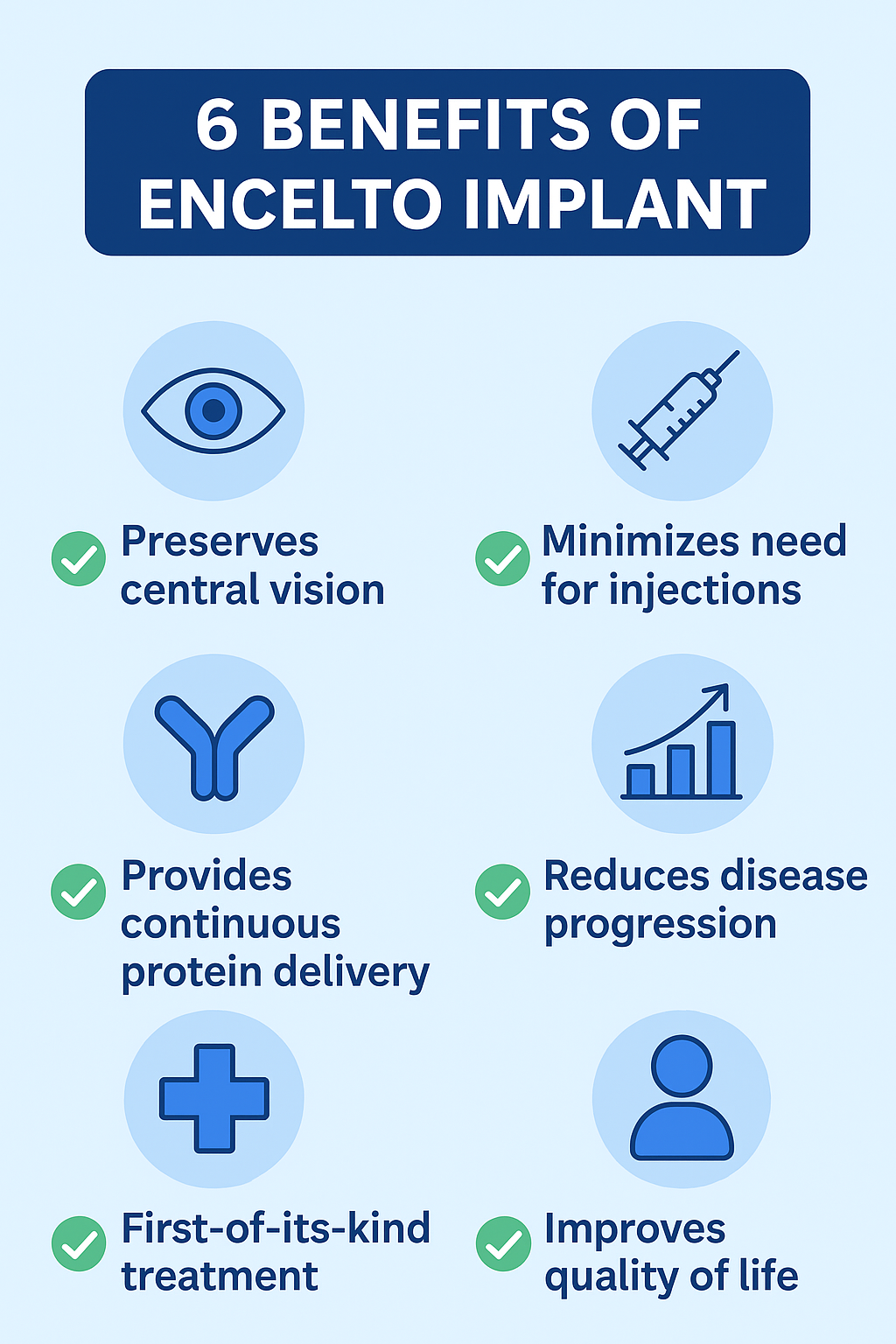
✅ Preserves central vision for the long term
✅ Reduces the need for repeated injections or repeated surgeries
✅ Delivers continuous protein to the retina
✅ Slows disease progression in MacTel Type 2
✅ First-of-its-kind treatment for a previously untreatable condition
For patients struggling with the fear of losing their vision, Encelto offers new hope.
Surgical Procedure for Encelto Implant
The implantation of Encelto is performed by a specialized ophthalmic surgeon.
- Pre-Surgery Evaluation
- Detailed eye examination, imaging, and eligibility assessment are conducted.
- Surgical Placement
- The device is implanted into the sub-retinal or periocular space through a minimally invasive surgical procedure.
- Post-Operative Care
- Patients are monitored for signs of infection, inflammation, or device malfunction.
- Regular follow-ups are required to assess visual outcomes.
Side Effects and Risks of Encelto Implant
Like all medical treatments, the it carries certain risks and side effects. Clinical trials reported:
- Mild to moderate eye irritation
- Inflammation or redness
- Risk of infection at the surgical site
- Rare cases of retinal detachment
- Possible device-related complications
Despite these risks, most patients tolerated the implant well, with benefits outweighing the risks.
Who is Eligible for Encelto Implant?
The Encelto implant is currently indicated for:
- ✅ Adults diagnosed with idiopathic macular telangiectasia type 2
- ✅ Patients showing signs of progressive central vision loss
- ✅ Individuals without major contraindications for ocular surgery
Cost and Accessibility of Encelto Implant
Since the therapy is newly approved, pricing and insurance coverage may vary. However:
- Advanced gene therapies like Encelto are typically expensive due to research and development costs.
- Patients should consult with ophthalmologists and insurance providers to explore coverage options.
- Patient-assistance programs are expected to launch soon after commercial availability.
Future of Encelto and Gene Therapy in Ophthalmology
The success of the Encelto implant is just the beginning. Experts predict:
- More cell-based therapies will emerge for retinal diseases.
- Personalized gene therapies could revolutionize vision care.
- The Encelto model may inspire treatments for conditions like age-related macular degeneration (AMD) and retinitis pigmentosa.
Patient Experience and Clinical Outcomes
Real-world evidence is expected to confirm clinical trial results. Patients receiving Encelto may experience:
- Slower vision deterioration
- Improved quality of life
- Reduced treatment burden compared to frequent eye injections
Testimonials from patients after FDA approval highlight renewed optimism in managing MacTel.
Conclusion
The Encelto implant marks a historic advancement in ophthalmology by offering the first effective therapy for MacTel Type 2. With its gene therapy-based approach, sustained protein delivery, and FDA approval in 2025, it opens new possibilities for millions of patients facing vision loss.
For those affected by MacTel, the Encelto implant is more than a treatment—it is a beacon of hope for preserving sight and improving quality of life.