Table of Contents
Introduction
Millions of people worldwide suffer from asthma, and severe and persistent allergic asthma is difficult to control with standard treatments. On March 7, 2025, the US Food and Drug Administration (FDA) approved Omlyclo® (omalizumab) as the first interchangeable biosimilar for Xolair® (omalizumab). This approval is a significant milestone in respiratory medicine, increasing access to life-saving treatments at a lower cost for patients. In this article, we will review the FDA approval of omalizumab, how it works, clinical benefits, side effects, dosage, and availability for patients and healthcare providers.
What is Omlyclo?
Omlyclo is a biologic medicine that is being developed as a biosimilar to Xolair® (omalizumab), a widely used medicine for the treatment of severe persistent allergic asthma.
A biosimilar is a biologic medicine that is identical to an already approved reference product (in this case, Xolair). Importantly, Omalizumab has no clinically meaningful differences in safety, purity, or efficacy compared to Xolair.
The FDA designation of “interchangeable” means that omalizumab can be used as an alternative to Xolair at the pharmacy level (subject to state laws), just like generic drugs.
FDA Approval of Omalizumab (2025)
On March 7, 2025, the FDA granted approval to omalizumab , making it the first interchangeable biosimilar to Xolair.
Why is this approval important?
- Expanded Patient Access – omalizumab provides a lower-cost alternative to Xolair, reducing treatment expenses.
- Therapeutic Equivalence – Omlyclo works the same way as Xolair, ensuring equal effectiveness.
- Market Competition – Encourages affordability and innovation in biologics.
This decision is expected to positively impact thousands of asthma patients who require omalizumab therapy.
How Does Omlyclo Work?
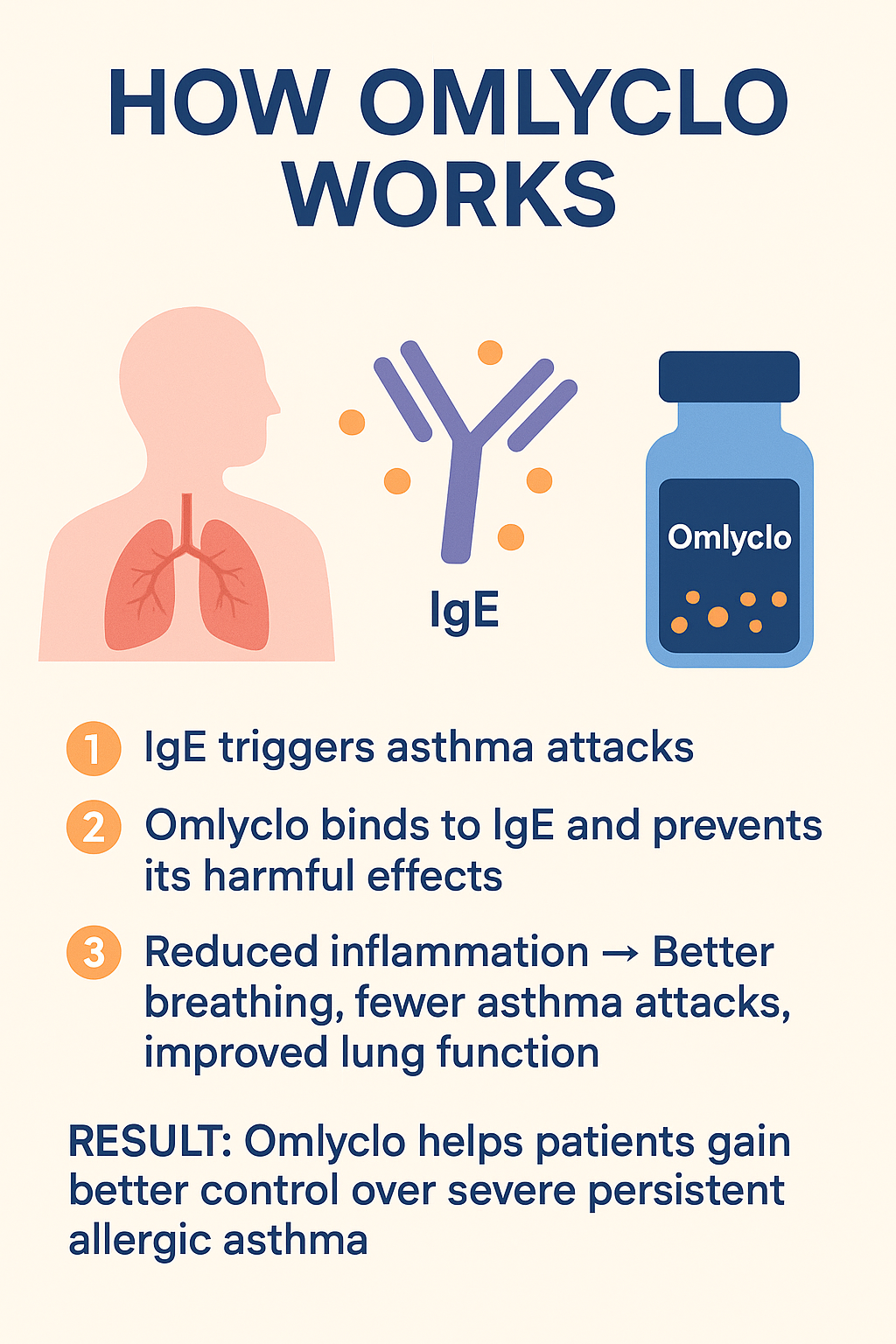
Asthma symptoms in patients with allergies are often caused by the antibody immunoglobulin E (IgE). High levels of IgE cause inflammation, narrowing, and chronic respiratory symptoms in the airways.
Omalizumab works by binding to free IgE in the blood. This prevents IgE from attaching to immune cells (mast cells and basophils), which otherwise release inflammatory mediators that cause asthma symptoms.
In simple terms:
1. IgE triggers asthma attacks in patients with allergies.
2. Omlyclo binds to IgE and blocks its harmful effects.
3. Inflammation is reduced → better breathing, fewer asthma attacks, improved lung function.
✅ Results: Omalizumab helps patients achieve better control of severe persistent allergic asthma.
Uses
Omalizumab is FDA-approved for:
- Treatment of moderate-to-severe persistent asthma in patients (≥6 years old) with positive skin test or in vitro reactivity to perennial aeroallergens, whose symptoms are not controlled with inhaled corticosteroids.
Note: Just like Xolair, Omlyclo is not intended for acute asthma attacks but for long-term management.
Benefits of Omlyclo
Omalizumab approval as a biosimilar brings multiple clinical and economic benefits.
Clinical Benefits
- Better asthma control in patients resistant to standard inhalers
- Fewer asthma exacerbations (severe attacks)
- Improved lung function (FEV1)
- Better quality of life and reduced hospital visits
Economic Benefits
- Lower cost compared to Xolair
- Greater insurance coverage potential
- Wider availability as an interchangeable biosimilar
Dosage and Administration of Omlyclo
Omalizumabis administered as a subcutaneous injection (under the skin).
General Guidelines:
- Frequency: Every 2–4 weeks (based on weight and IgE levels)
- Location: Upper arm, thigh, or abdomen
- Healthcare Setting: Typically administered in a clinic or hospital due to risk of allergic reactions
Patients must undergo baseline IgE testing to determine the correct dosage.
Side Effects
Like Xolair,omalizumab may cause side effects.
Common Side Effects:
- Injection site reactions (pain, redness, swelling)
- Headache
- Fatigue
- Joint or muscle pain
Rare but Serious Side Effects:
- Anaphylaxis (severe allergic reaction) – requires immediate medical attention
- Increased risk of parasitic infections
- Rare cardiovascular or cerebrovascular events
Healthcare providers closely monitor patients after injections to ensure safety.
Safety Precautions and Warnings
Before starting omalizumab , patients should inform their doctor if they have:
- History of anaphylaxis or severe allergic reactions
- Cardiovascular disease
- Parasitic infections
- Current pregnancy or breastfeeding
Black Box Warning: Like Xolair, omalizumab carries a warning for potential anaphylaxis. Patients must remain under medical observation for a period after injections.
Omlyclo vs. Xolair: What’s the Difference?
Since Omlyclo is a biosimilar, it is nearly identical to Xolair.
| Feature | Omlyclo (Biosimilar) | Xolair (Reference Drug) |
| Active Ingredient | Omalizumab | Omalizumab |
| FDA Approval | March 7, 2025 | 2003 |
| Indication | Severe allergic asthma | Severe allergic asthma |
| Cost | Lower | Higher |
| Interchangeable status | Yes | Not applicable |
✅ Bottom line: Omalizumab offers the same benefits as Xolair, but at a lower price.
Cost and Availability of Omlyclo
Omalizumab approval means more patients will have access to life-changing treatment.
- Price: Expected to be 20–30% lower than Xolair
- Insurance: Likely to be included in formulary lists given its interchangeable status
- Distribution: Specialty pharmacies and healthcare centers
With lower costs and FDA approval, omalizumab will help reduce financial barriers for asthma patients.
Future Outlook
Omalizumabapproval may pave the way for:
- Expansion into other indications where Xolair is used (e.g., chronic idiopathic urticaria, nasal polyps)
- More biosimilars entering the market for respiratory conditions
- Enhanced competition leading to affordable biologic therapies
This milestone highlights the growing role of biosimilars in healthcare affordability.
Conclusion
The approval of Omlyclo® (omalizumab) as the first interchangeable biosimilar to Xolair® on March 7, 2025, represents a major advance in asthma care. It provides similar clinical benefits at a lower cost, improving accessibility for patients with severe persistent allergic asthma. Omlyclo not only offers hope for better asthma control but also lays the foundation for more biosimilars in respiratory medicines. For patients who still struggle with asthma after using an inhaler, Omlyclo could be a game-changing therapy.
Frequently Asked Questions (FAQs)
1. What is omalizumab used for?
2. Is Omlyclo the same as Xolair?
3. How is omalizumab given?
4. Is omalizumab cheaper than Xolair?
5. What are the side effects of omalizumab ?
6. Can Omlyclo be substituted for Xolair at the pharmacy?
7. Who should not take omalizumab ?
Disclaimer:
This content is for educational and informational purposes only and should not be considered medical advice. Always consult a qualified healthcare professional for diagnosis, treatment, or any questions regarding your health condition.

