Table of Contents
Introduction
On February 11, 2025, the U.S. Food and Drug Administration (FDA) announced the approval of mirdametinib, marketed under the brand name Gomecli. This approval marks a historic milestone in the management of neurofibromatosis type 1 (NF1)-associated symptomatic plexiform neurofibromas (PNs) – a rare and challenging condition.
The mirdametinib FDA approval is a significant advancement because it offers a non-surgical treatment option for adults and children aged two years and older who suffer from tumors that cannot be completely removed by surgery. Patients with NF1-associated plexiform neurofibromas have long struggled with limited treatment options, making this approval both promising and transformative.
In this article, we will explore everything you need to know about the mirdametinib FDA approval, its clinical uses, mechanism of action, dosage, benefits, side effects, and its broader implications for patients and healthcare providers.
What is Mirdametinib?
Mirdametinib is an oral MEK1/2 inhibitor specifically developed to target abnormal cell signaling involved in tumor growth in NF1 patients. Unlike surgical approaches, which often cannot completely remove plexiform neurofibromas due to their location and spread, mirdametinib provides a pharmacological alternative that reduces tumor burden and improves quality of life.
It is sold under the trade name Gomekli and is now mirdametinib FDA approval as a precision medicine for NF1 patients of various ages.
Understanding NF1 and Plexiform Neurofibromas
To understand the implications of the mirdametinib FDA approval , it is important to understand what neurofibromatosis type 1 (NF1) is.
• NF1 is a rare genetic disorder caused by mutations in the NF1 gene, which regulates cell growth.
• Patients often develop plexiform neurofibromas (PNs) – large, complex tumors that grow near nerves.
• These tumors can cause pain, deformity, nerve compression, movement problems, and functional impairment.
• Surgery is risky and often incomplete due to the location and size of the tumor.
Mirdametinib offers hope for this patient community by providing a non-invasive treatment option with clinical evidence of effectiveness.
Mirdametinib FDA Approval: Key Details
- Approval Date: February 11, 2025
- Brand Name: Gomekli
- Indication: Treatment of adults and children aged ≥2 years with NF1-associated symptomatic plexiform neurofibromas not amenable to complete surgical resection
- Regulatory Pathway: Standard FDA approval following positive clinical trial results
This approval is especially important because it expands treatment options for both pediatric and adult populations, addressing a long-standing unmet medical need.
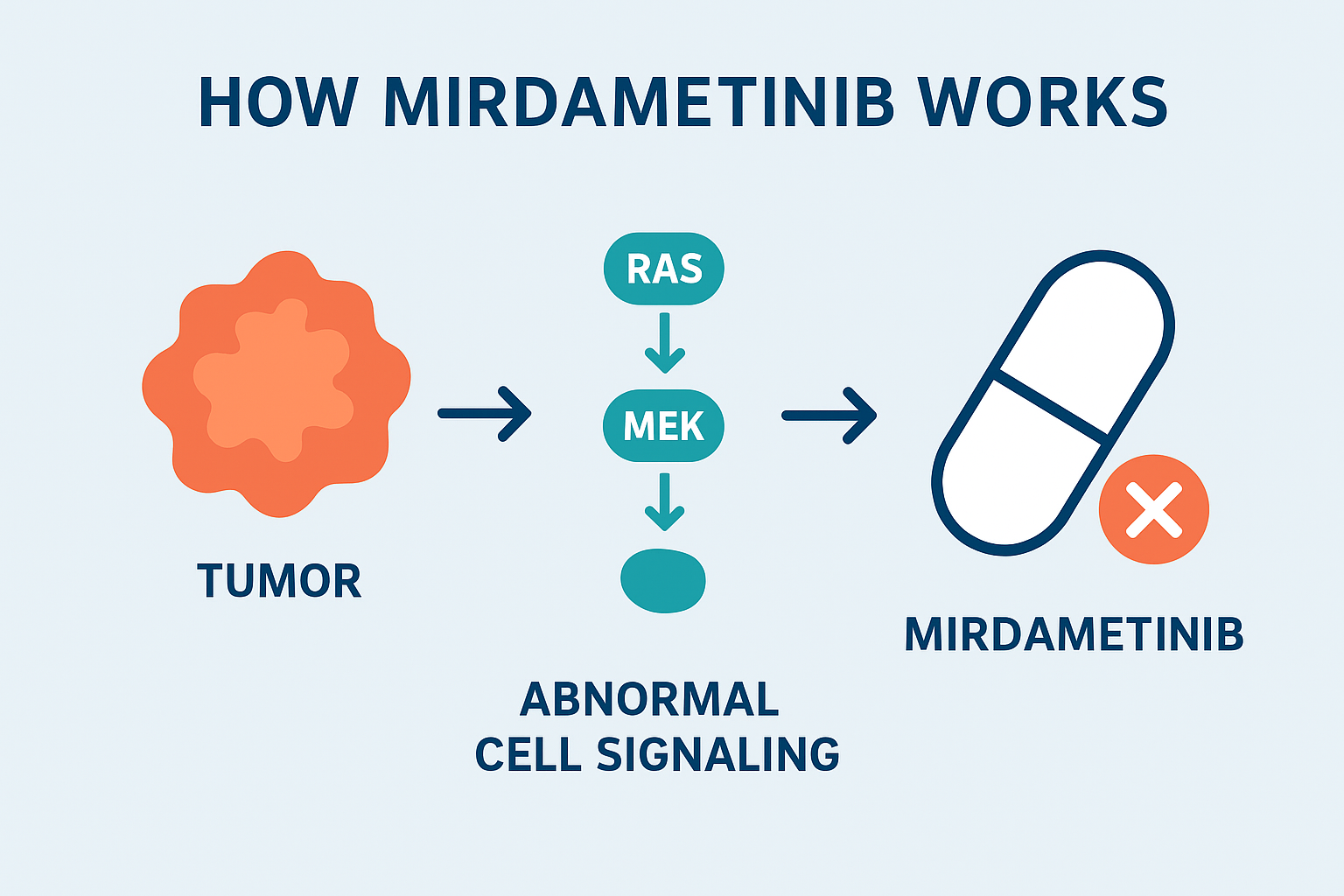
How Does Mirdametinib Work? (Mechanism of Action)
Mirdametinib works by inhibiting MEK1 and MEK2, two proteins in the RAS/MAPK signaling pathway.
• In NF1, mutations cause uncontrolled activation of this pathway, which leads to tumor growth.
• By inhibiting MEK1/2, mirdametinib reduces abnormal signaling, which slows or stops tumor growth.
This mechanism makes it a targeted therapy, different from traditional chemotherapy or radiation therapies.
Clinical Trials Leading to FDA Approval
The FDA’s decision was based on robust clinical trial evidence demonstrating the safety and efficacy of mirdametinib.Benefits of Mirdametinib
Mirdametinib FDA approval offers multiple benefits:
Scientific Advancement – Represents progress in precision medicine for rare genetic disorders.
Non-Surgical Option – Provides treatment for tumors that cannot be surgically removed.
Applicable to All Ages – Approved for both adults and children ≥2 years.
Symptom Relief – Reduces tumor-related pain, disfigurement, and functional impairment.
Improved Quality of Life – Patients can manage symptoms without repeated surgeries.
Key Findings:
• Tumor shrinkage: Many patients experienced significant reductions in tumor size, while others experienced partial responses.
• Symptom improvement: Patients reported reduced pain, improved function, and improved quality of life.
• Pediatric benefit: The trials included children as young as 2 years old, demonstrating its safety in people of all ages.
These results convinced regulators that mirdametinib was effective and tolerable, paving the way for its approval.
Benefits of Mirdametinib
Mirdametinib FDA approval offers multiple benefits:
- Non-Surgical Option – Provides treatment for tumors that cannot be surgically removed.
- Applicable to All Ages – Approved for both adults and children ≥2 years.
- Symptom Relief – Reduces tumor-related pain, disfigurement, and functional impairment.
- Improved Quality of Life – Patients can manage symptoms without repeated surgeries.
- Scientific Advancement – Represents progress in precision medicine for rare genetic disorders.
Dosage and Administration
- Form: Oral capsules
- Administration: Taken twice daily
- Weight-Based Dosing: Pediatric dosing is adjusted according to body surface area.
- Treatment Duration: Continued until disease progression or unacceptable toxicity
Healthcare providers must tailor dosage to each patient while monitoring for adverse events.
Possible Side Effects of Mirdametinib
Like all targeted therapies, mirdametinib may cause side effects. Patients should be closely monitored during treatment.
Common Side Effects:
- Fatigue
- Skin rash
- Diarrhea
- Nausea
- Abdominal pain
- Muscle or joint pain
Serious Risks:
- Heart problems (reduced ejection fraction)
- Eye disorders (retinal issues)
- Liver enzyme elevations
Healthcare providers must assess risks and adjust treatment if necessary.
Comparison with Other NF1 Treatments
Before the mirdametinib FDA approval , treatment options were extremely limited. The only FDA-approved drug for NF1-associated plexiform neurofibroma was selumetinib (Coselugo), which was approved in 2020.
Mirdametinib expands the arsenal by:
• Offering alternative MEK inhibitors with different safety/efficacy data
• Covering pediatric and adult patients
• Increasing treatment personalization
This provides doctors and patients with more choice and flexibility.
Patient Perspective: Why This Approval Matters
For patients and families affected by NF1, the FDA approval of mirdametinib is life-changing. Many have endured years of surgeries, complications, and limited treatment options. With this approval:
• Children as young as 2 can start treatment sooner.
• Adults who previously did not have an FDA-approved option now have access.
• Families gain hope for better long-term management.
Market Impact and Accessibility
Following mirdametinib FDA approval , Gomekli will soon be available in the US market. Availability considerations include:
• Insurance coverage: As an FDA-approved therapy for a rare disease, it may be included in rare-disease treatment plans.
• Patient assistance program: The manufacturer is expected to offer a financial assistance program.
Global outlook: Approval in other regions (EU, Asia) may follow US regulatory approval.
Future Research Directions
The mirdametinib FDA approval is just the beginning. Ongoing research could explore:
• Long-term safety in pediatric patients
• Combination therapy with other targeted drugs
• Expanded indications for other tumor types
This lays the foundation for future innovations in neuro-oncology and genetic disorder management.
Conclusion
The mirdametinib FDA approval is a significant milestone in the treatment of neurofibromatosis type 1-associated plexiform neurofibroma. For decades, patients had limited and risky treatment options, but now both children and adults can benefit from targeted, effective, and nonsurgical therapies. This milestone not only represents a victory for the NF1 community, but also highlights the advancement of precision medicine to address rare genetic disorders. With ongoing research and widespread availability, mirdametinib (Gomekli) is poised to transform patient care and bring hope to countless families around the world.
Frequently Asked Questions (FAQs)
1. What is mirdametinib used for?
2. When did the FDA approve mirdametinib?
3. What is the brand name of mirdametinib?
4. How does mirdametinib work?
5. What are the common side effects of mirdametinib?
6. Is mirdametinib safe for children?
7. How does mirdametinib compare to selumetinib?
Disclaimer:
This content is for educational and informational purposes only and should not be considered medical advice. Always consult a qualified healthcare professional for diagnosis, treatment, or any questions regarding your health condition.

