Table of Contents
Introduction
When preparing for allogeneic stem cell transplantation – particularly in cases of acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) – having a safe, effective preparation regimen is of utmost importance. Enter GRAFAPEX, the brand name for treosulfan, a novel alkylating agent that is gaining attention for its attractive combination of efficacy and tolerability. Approved by the FDA in early 2025, GRAFAPEX, in combination with fludarabine, represents a modern approach to conditioning prior to transplantation.
This post delves into what makes GRAFAPEX unique – its mechanism of action, clinical performance, safety, and role in contemporary transplant protocols – to help patients, families, and medical professionals understand its benefits and considerations.
What Is GRAFAPEX?
GRAFAPEX (treosulfan) is an alkylating chemotherapy agent used as a conditioning regimen in combination with fludarabine for allogeneic hematopoietic stem cell transplantation (alloHSCT) in patients one year of age and older with AML or MDSS. As of January 2025, GRAFAPEX has received FDA approval for this indication, marking its emergence as a new key component in transplant conditioning.
How Does Treosulfan Work?
As an alkylating agent, GRAFAPEX disrupts DNA structure in rapidly dividing cells – particularly hematopoietic stem cells – by adding alkyl groups to the DNA, which leads to cell death. Combined with fludarabine, it provides the myelosuppression and immunosuppression necessary for successful transplant engraftment and anti-tumor activity.
FDA Approval: What the Data Show
On January 21-22, 2025, the FDA approved treosulfan and fludarabine for preparative regimens in adult and pediatric patients (≥1 year of age) with AML or MDS. The approval was based on results from a large randomized Phase III trial – MC-FludT.14/L Trial II (NCT00822393) – in which GRAFAPEX was compared to busulfan, both in combination with fludarabine.
Key efficacy results include:
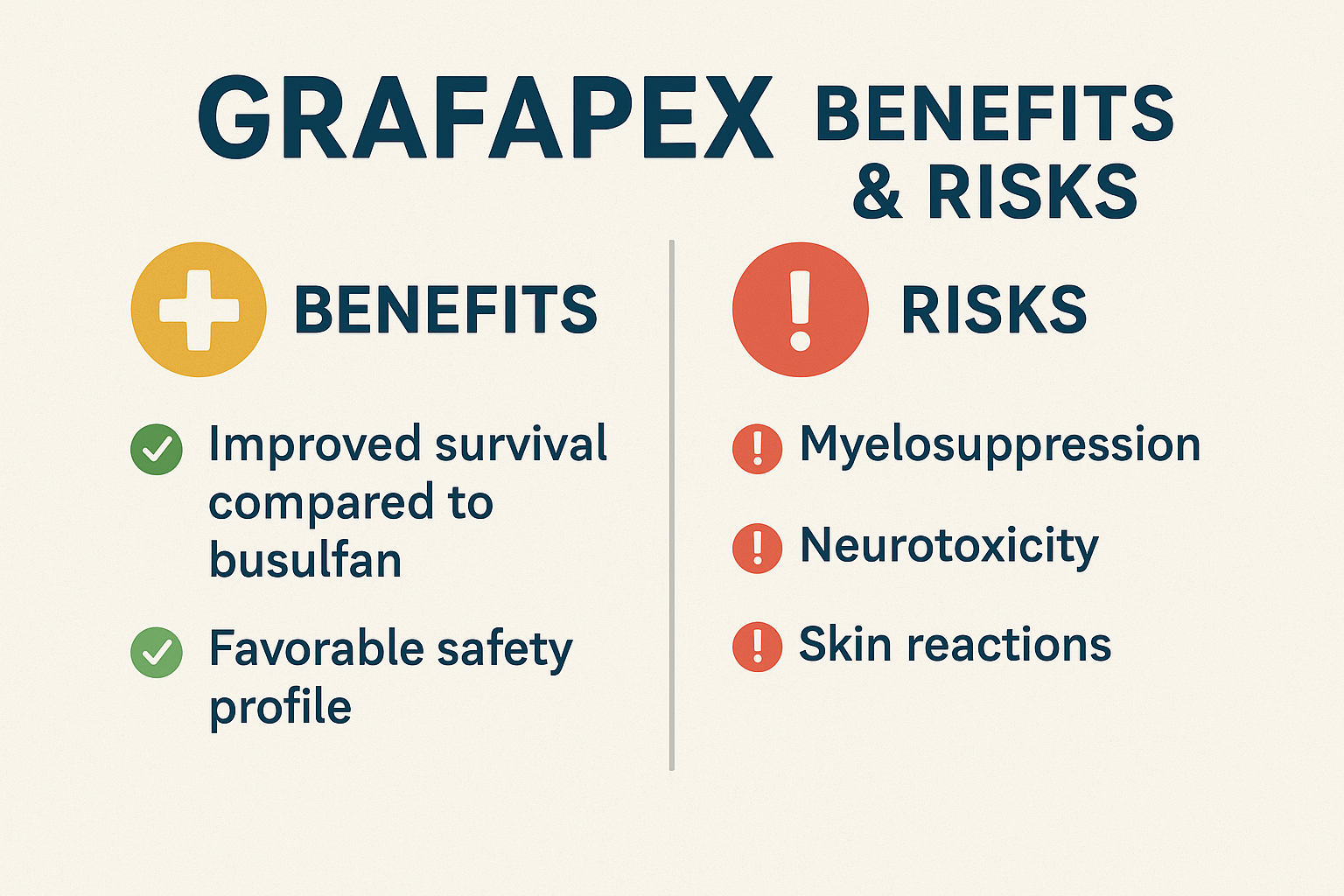
• Overall survival (OS) hazard ratio (HR) 0.67 (95% CI: 0.51–0.90) favoring GRAFAPEX in the overall randomized population, with benefits seen in both AML (HR 0.73) and MDS (HR 0.64).
• In a broader real-world context, event-free survival at 2 years: 64% for GRAFAPEX versus 50.4% for busulfan (HR 0.65; p < 0.0051).
Safety Profile & Clinical Considerations
Treosulfan is potent—and it has serious safety concerns:
• Boxed Warning (Myelosuppression): Treosulfan causes severe, prolonged pancytopenia; stem cell infusion is required to prevent fatal outcomes. Daily monitoring of blood counts is essential.
• Neurological Risk: Seizures have been reported; prophylactic clonazepam may be considered for high-risk patients, including young children.
• Skin Reactions: Rash and dermatitis—especially when sodium-bicarbonate hydration is used—need careful skin care and dressing changes; diaper rash may occur in young children.
• Injection Site Necrosis: Ensure the integrity of the vein during infusion to avoid local tissue damage in the event of extravasation.
• Secondary Malignancy Risk: Like other alkylators, treosulfan is carcinogenic/genotoxic. Long-term monitoring is advised, especially in patients with DNA repair disorders.
• Embryo-fetal toxicity: GRAFAPEX may cause fetal harm. Effective contraception is required – during treatment and for 6 months after treatment in women, 3 months in men.
• Specific adverse events: The most common adverse events (≥20% incidence) included musculoskeletal pain, stomatitis, fever, nausea, edema, infection, and vomiting. Significant increases in GGT, bilirubin, ALT, AST, and creatinine were also reported.
Practical Use: Dosage & Administration
The recommended regimen:
• GRAFAPEX (treosulfan): 10 g/m2 injection once daily on days -4, -3 and -2 before transplantation.
• Fludarabine: 30 mg/m2 injection daily on days -6 to -2.
• Stem cell injection occurs on day 0.
• Preparation instructions: Available in lyophilized vials (1 g and 5 g). Reconstitute in saline, 5% dextrose or sterile water (not recommended for children only). Aim for a concentration of approximately 0.05 g/ml.
• The reconstituted solution is stable for up to 24 hours at 20-25 °C (no refrigeration) unless a precipitate is observed.
• Administer slowly over 2 hours with continuous monitoring for extravasation.
Why Treosulfan Matters Today
Innovation designed for vulnerable patients
GRAFAPEX expands conditioning options, particularly for elderly or co-morbid patients for whom intensive protocols may be too toxic.
Excellent survival and safety profile
Improved OS and EFS compared to busulfan, with manageable side effects, make GRAFAPEX a preferred option in many transplant settings.
Orphan drug and market potential
By receiving orphan designation in the US, GRAFAPEX benefits from regulatory exclusivity. Medexus (US commercial rights holder) expects to generate over US$100M in revenue over five years following launch, which is planned for early to mid-2025.
Key Takeaways for Patients & Clinicians
| Consideration | Insight |
| Indication | AML or MDS in patients ≥1 year undergoing alloHSCT |
| Strength | Improved survival vs. busulfan; favorable toxicity profile |
| Risks | Myelosuppression, neuro, skin, injection-site, malignancy |
| Administration | Days –4 to –2 (treosulfan), short 2h infusions, combo with fludarabine |
| Best Use Cases | Older/comorbid patients, those needing more tolerable conditioning |
| Market Trajectory | US benefit exclusivity; commercial rollout underway |
Conclusion
GRAFAPEX is a milestone in stem cell transplant conditioning. With FDA approval in January 2025, robust trial data, and a structured administration protocol, it is poised to improve outcomes for many AML and MDS patients—especially those who are not amenable to conventional therapies.
By balancing efficacy and tolerability, Treosulfan provides a modern, patient-sensitive conditioning approach that shapes the transplant strategy. Whether you are a healthcare provider guiding therapy or a patient/family navigating treatment, it is critical to understand the role, risks, and regimen of GRAFAPEX.
FAQs
1. What is GRAFAPEX used for?
2. How does treosulfan work?
3. What are the side effects of GRAFAPEX?
4. Is GRAFAPEX safer than busulfan?
5. Who should not use treosulfan?
Disclaimer
This article is for educational purposes only and is not a substitute for medical advice. Always consult your healthcare provider before starting or changing any treatment, including GRAFAPEX.