Table of Contents
1.Introduction: Shifting the Paradigm in Lung Cancer Treatment
Non-small cell lung cancer (NSCLC) remains a formidable challenge in oncology, particularly for patients whose tumors harbor activating mutations in the epidermal growth factor receptor (EGFR) gene. For years, EGFR tyrosine kinase inhibitors (TKIs) have served as the cornerstone of first-line therapy, offering significant improvements over traditional chemotherapy. However, the inevitable development of resistance and the persistent challenge of treating central nervous system (CNS) metastases have driven the continuous search for more effective and durable treatment strategies.
The recent introduction of the combination therapy involving amivantamab (Rybrevant) and lazertinib (Lazcluze) marks a pivotal moment in this therapeutic landscape and signals a potential new standard of care. This innovative regimen offers a chemotherapy-free approach that delivers deeper, more sustained clinical benefit.
The combination of Rybrevant, a bispecific antibody, and Lazcluze, a third-generation EGFR TKI, represents a strategic multi-pronged attack on tumor biology. This dual-targeting approach is designed not only to maximize initial tumor response but also to proactively address common mechanisms of acquired resistance. The strong clinical data supporting this regimen, particularly from the landmark Phase 3 MARIPOSA trial, underscores the transformative potential of the Rybrevant Lazcluze MOA.
2.FDA Approval and the Landmark MARIPOSA Trial
On August 19, 2024, the U.S. Food and Drug Administration granted approval for lazertinib in combination with amivantamab for the first-line treatment of adult patients with locally advanced or metastatic NSCLC harboring EGFR exon 19 deletions or exon 21 L858R substitution mutations.
This regulatory milestone was driven by robust evidence from the Phase 3 MARIPOSA trial, which demonstrated superior efficacy of the combination compared with established standard-of-care therapy.
The MARIPOSA Trial: Setting a New Benchmark
The MARIPOSA trial was a randomized, head-to-head Phase 3 study enrolling patients with newly diagnosed, advanced EGFR-mutated NSCLC. Participants were assigned to one of three treatment arms:
- Rybrevant plus Lazcluze
- Osimertinib monotherapy
- Lazertinib monotherapy
The primary endpoint was progression-free survival (PFS).
Key Efficacy Outcomes
The Rybrevant Lazcluze combination demonstrated a statistically significant and clinically meaningful improvement in progression-free survival compared with osimertinib monotherapy. This finding is particularly notable given that osimertinib had been regarded as the most effective third-generation EGFR TKI available.
In addition to improved PFS, the combination showed a favorable trend toward improved overall survival, reducing the risk of death compared with osimertinib alone. These outcomes collectively elevate expectations for first-line treatment efficacy in EGFR-mutated NSCLC.
CNS Activity and Response Rates
Central nervous system involvement remains a major concern in EGFR-mutated NSCLC. Lazcluze, as a CNS-penetrant TKI, was expected to enhance intracranial disease control. MARIPOSA trial data confirmed this hypothesis, demonstrating robust intracranial response rates and prolonged CNS progression-free survival.
The overall response rate for the combination was high, with many patients experiencing rapid and deep tumor shrinkage. This comprehensive efficacy profile—including superior systemic control, CNS protection, and durable responses—represents the clinical expression of the Rybrevant Lazcluze MOA.
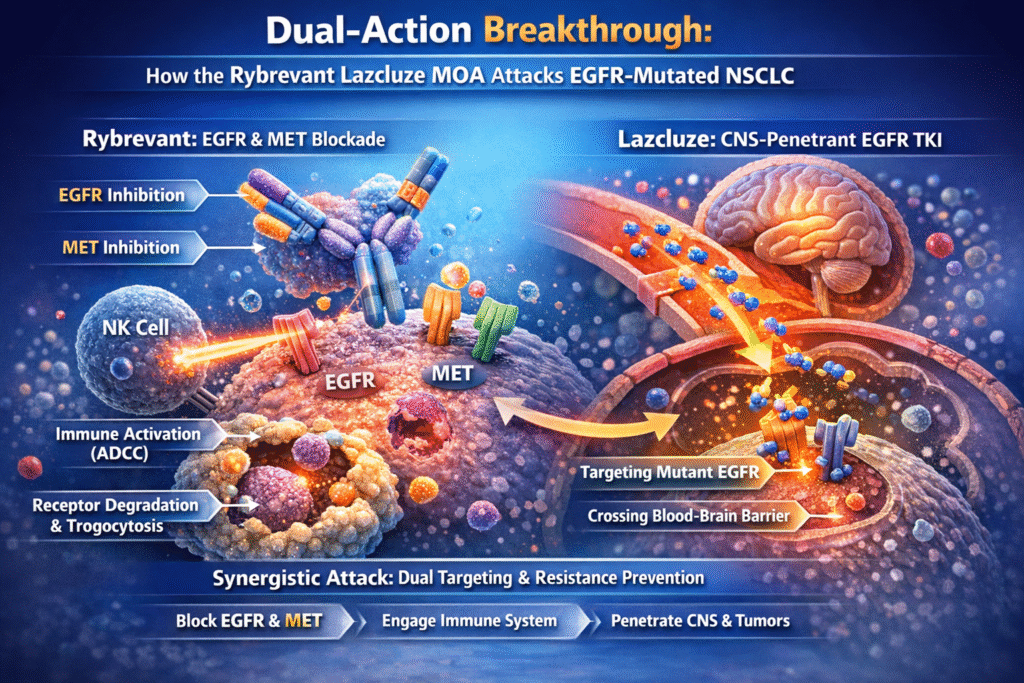
3.Unpacking the Rybrevant Lazcluze MOA: A Dual-Action Strategy
The innovation of this regimen lies in its complementary and layered mechanism of action. The Rybrevant Lazcluze MOA simultaneously targets extracellular receptors, intracellular signaling pathways, and immune-mediated tumor destruction, resulting in a more complete blockade of oncogenic signaling and resistance pathways.
Rybrevant (Amivantamab): The Bispecific Antibody
Rybrevant is a fully human bispecific IgG1 antibody designed to target both the epidermal growth factor receptor (EGFR) and the mesenchymal-epithelial transition (MET) receptor.
Mechanistic Components
- Dual receptor targeting
Rybrevant binds to the extracellular domains of EGFR and MET. MET pathway activation is a well-established mechanism of resistance to EGFR TKIs. By inhibiting both pathways simultaneously, Rybrevant prevents tumor escape via MET-driven signaling. - Immune cell–directing activity
Rybrevant possesses enhanced antibody-dependent cellular cytotoxicity due to its low-fucose IgG1 structure. Once bound to tumor cells, it recruits natural killer cells and other immune effectors, promoting immune-mediated tumor cell destruction. - Receptor degradation and trogocytosis
Rybrevant promotes internalization and degradation of EGFR and MET receptors. It also induces trogocytosis, a process whereby immune cells physically extract tumor cell membrane components, leading to cellular destruction.
Lazcluze (Lazertinib): The Third-Generation EGFR TKI
Lazcluze is a highly selective and irreversible third-generation EGFR tyrosine kinase inhibitor.
Key Functional Attributes
- Selective inhibition
Lazcluze selectively inhibits EGFR exon 19 deletions and L858R mutations while sparing wild-type EGFR, reducing off-target toxicity such as severe skin and gastrointestinal adverse effects. - CNS penetration
Lazcluze effectively crosses the blood–brain barrier, enabling sustained EGFR inhibition within brain metastases. This feature is critical for improving both survival and quality of life in patients with CNS involvement.
4.Synergy of the Rybrevant Lazcluze MOA
| Component | Target | Mechanism of Action | Role in Combination |
| Rybrevant | Extracellular EGFR & MET | ADCC, receptor degradation, trogocytosis | Immune engagement and resistance prevention |
| Lazcluze | Intracellular mutant EGFR | Irreversible kinase inhibition | CNS control and intracellular blockade |
This synergistic approach ensures tumor suppression from multiple biological angles, delivering deeper and more durable responses.
5.Safety Profile and Adverse Event Management
While offering superior efficacy, the Rybrevant Lazcluze MOA is associated with a distinct safety profile that requires proactive management.
Common Adverse Reactions
- Dermatologic toxicity including rash, paronychia, pruritus, and dry skin
- Infusion-related reactions, particularly during the first dose
- Gastrointestinal symptoms such as diarrhea, nausea, and constipation
- Fatigue, musculoskeletal pain, stomatitis, and peripheral edema
Serious Warnings and Precautions
| Risk | Description | Management |
| Venous thromboembolism | Early risk of DVT and pulmonary embolism | Prophylactic anticoagulation for first four months |
| Interstitial lung disease | Potentially fatal pneumonitis | Immediate discontinuation upon suspicion |
| Infusion-related reactions | Includes anaphylaxis | Mandatory premedication and infusion control |
| Severe dermatologic toxicity | Rare SJS/TEN | Dose interruption or discontinuation |
| Ocular toxicity | Keratitis and dry eye | Baseline and follow-up ophthalmologic exams |
6.Drug Interactions with Rybrevant Lazcluze MOA
Lazcluze (Lazertinib) and CYP3A4
Lazertinib is primarily metabolized via CYP3A4.
| Interacting Drug Class | Effect | Recommendation |
| CYP3A4 inducers | Reduced plasma levels | Avoid concomitant use |
| CYP3A4 inhibitors | Increased toxicity | Avoid or reduce dose |
| CYP3A4 substrates | Elevated exposure | Monitor adverse effects |
| P-gp/BCRP substrates | Increased drug levels | Close monitoring |
7.Rybrevant Drug Interaction Profile
Rybrevant is not metabolized through cytochrome P450 enzymes and has minimal interaction potential. Most clinically relevant interactions originate from Lazcluze.
Practical Advantage: Subcutaneous Rybrevant Formulation
The approval of a subcutaneous formulation of Rybrevant (Rybrevant Faspro) significantly improves treatment practicality. This formulation allows administration in approximately five minutes, reducing infusion-related burden and improving patient convenience while maintaining therapeutic efficacy.
Conclusion: The Future of EGFR-Mutated NSCLC
The Rybrevant Lazcluze combination represents a major advancement in first-line EGFR-mutated NSCLC therapy. By combining extracellular receptor blockade, immune-mediated cytotoxicity, and intracellular kinase inhibition, the Rybrevant Lazcluze MOA establishes a new benchmark for treatment durability and disease control.
With strong clinical evidence, manageable safety considerations, and improved administration options, this dual-action regimen defines the future direction of targeted therapy in EGFR-driven lung cancer.

