Table of Contents
The FDA approval of Plozasiran represents a landmark achievement in the management of rare lipid disorders, particularly Familial Chylomicronemia Syndrome (FCS). For decades, patients with extremely high triglyceride levels had limited treatment options, relying primarily on dietary restrictions and supportive care. The approval of Plazomicin provides a novel, targeted, and science-based therapeutic approach that addresses the underlying cause of triglyceride accumulation.
This article provides a comprehensive overview of Plozasiran FDA approval, its mechanism of action, clinical significance, safety profile, dosing considerations, and future implications for patients, clinicians, pharmacists, and the pharmaceutical industry.
Understanding Familial Chylomicronemia Syndrome (FCS)
Familial chylomicronemia syndrome (FCS) is a rare genetic metabolic disorder characterized by extremely elevated triglyceride levels, often exceeding 1000 mg/dL and sometimes even reaching over 2000 mg/dL. This condition is caused by defects in genes involved in triglyceride metabolism, particularly those affecting the function of lipoprotein lipase.
Patients with FCS typically experience the following symptoms:
• Frequent episodes of acute pancreatitis
• Severe abdominal pain
• Fatigue and cognitive impairment
• Eruptive xanthomas
• Lipemia retinalis
Even with strict adherence to a very low-fat diet, many patients continue to have dangerously high triglyceride levels. This unmet medical need led to the FDA approval of plozasiran.
What Is Plozasiran?
Plozasiran is a novel RNA interference (RNAi)-based treatment designed to reduce the production of apolipoprotein C-III (ApoC-III), a key regulator of triglyceride metabolism. ApoC-III inhibits the breakdown and clearance of triglyceride-rich lipoproteins from the body. Elevated levels of ApoC-III significantly contribute to hypertriglyceridemia.
By targeting ApoC-III at the genetic level, plozasiran provides a precision-based approach rather than merely treating the symptoms. The FDA approval received by plozasiran demonstrates the clinical significance of this innovative strategy.
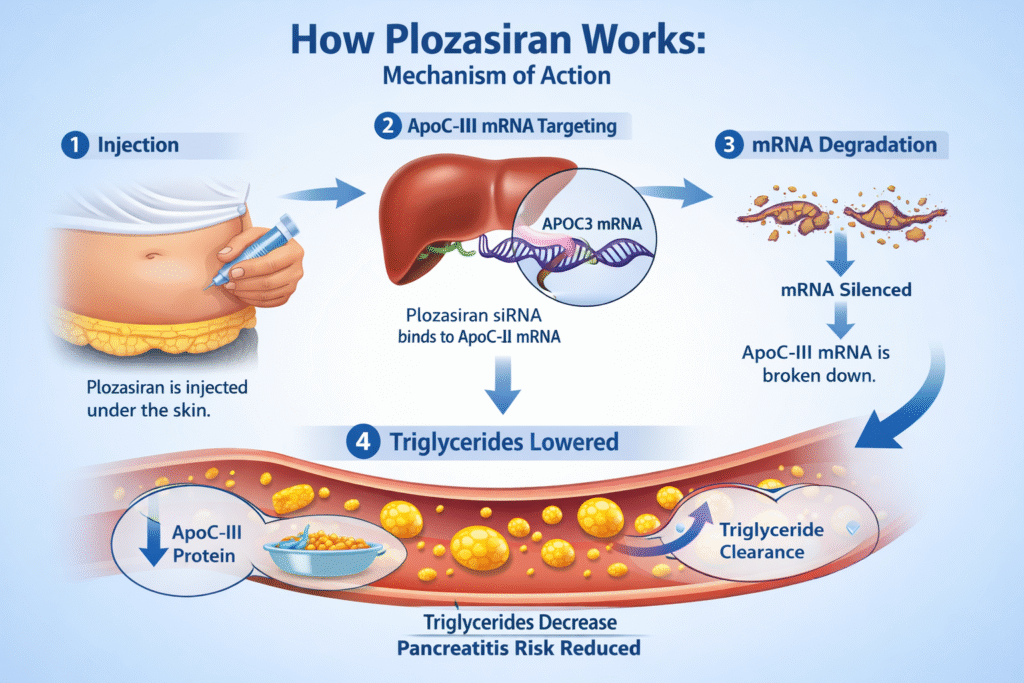
Mechanism of Action of Plozasiran
The mechanism of action of Plozasiran is based on RNA interference technology. After administration via subcutaneous injection, Plozasiran is delivered specifically to liver cells using a targeted molecular structure.
Once inside the liver cells:
1. Plozasiran binds to ApoC-III messenger RNA.
2. The RNA-induced silencing complex degrades the targeted mRNA.
3. The synthesis of ApoC-III protein is significantly reduced.
4. Triglyceride clearance improves.
5. Blood triglyceride levels are substantially lowered.
This highly selective mechanism of action explains why the FDA approval of Plozasiran is a significant step towards disease-modifying treatments in lipid disorders.
Plozasiran FDA Approval: Regulatory Overview
Plozasiran has received FDA approval for use as an adjunct to dietary management in adults with familial chylomicronemia syndrome. This approval was based on robust clinical trial data demonstrating significant triglyceride reduction and an acceptable safety profile.
Key features of the approval include:
• Indication limited to adult patients with FCS only
• Use in conjunction with strict dietary fat restrictions
• Administered via subcutaneous injection
• Periodic dosing rather than daily treatment
The FDA recognized the urgent need for effective treatments in this extremely rare population, making the FDA approval of plozasiran a patient-centered regulatory decision.
Clinical Trial Evidence Supporting Approval
The approval of plozasiran was supported by advanced-phase clinical trials that evaluated efficacy, safety, and tolerability in patients with genetically confirmed FCS.
Efficacy Outcomes
Clinical studies demonstrated:
- Substantial and sustained reductions in triglyceride levels
- Rapid onset of action after dosing
- Consistency across patient subgroups
- Maintenance of triglyceride reduction over time
These outcomes were central to the plozasiran FDA approval, as triglyceride reduction is directly linked to pancreatitis risk reduction.
Clinical Relevance
Lowering triglyceride levels in FCS patients is not merely a laboratory achievement. It translates into:
- Fewer pancreatitis episodes
- Reduced hospitalization rates
- Improved quality of life
- Less dietary burden
These real-world benefits strengthened the case for plozasiran FDA approval.
Safety and Tolerability Profile
Safety evaluation was a critical component of the regulatory review. Overall, plozasiran demonstrated a favorable benefit-risk profile.
Common Adverse Effects
Reported adverse events included:
- Injection-site reactions
- Mild flu-like symptoms
- Transient laboratory abnormalities
Serious Adverse Events
Serious adverse events were uncommon and generally related to underlying disease rather than treatment. Most patients were able to continue therapy without discontinuation.
The acceptable safety profile contributed significantly to the plozasiran FDA approval, especially considering the severity of untreated FCS.
Dosing and Administration
Plozasiran is administered via subcutaneous injection. Unlike daily oral therapies, plozasiran follows a periodic dosing schedule, which enhances patient adherence.
Key Administration Points
- Administered by healthcare professionals or trained patients
- Given at extended intervals
- No complex infusion requirements
- Compatible with outpatient management
The convenience of dosing is another factor that supports the positive impact of the plozasiran FDA approval.
Role of Diet Alongside Plozasiran
Although plozasiran is highly effective, dietary management remains essential. Patients are advised to continue:
- Very low-fat diets
- Avoidance of alcohol
- Careful monitoring of caloric intake
The plozasiran FDA approval specifies use as an adjunct therapy, reinforcing the importance of comprehensive disease management.
Impact on Patients’ Quality of Life
One of the most meaningful aspects of the plozasiran FDA approval is its potential to transform daily life for patients with FCS.
Patients may experience:
- Reduced fear of pancreatitis attacks
- Greater dietary flexibility
- Improved physical and mental well-being
- Enhanced social participation
For a condition that previously imposed severe lifestyle restrictions, the psychological benefit of plozasiran cannot be overstated.
Significance for Physicians and Pharmacists
Healthcare professionals play a crucial role in maximizing the benefits of plozasiran.
For Physicians
- Accurate diagnosis of FCS is essential
- Patient selection must follow approved indications
- Ongoing monitoring of triglyceride levels is required
For Pharmacists
- Education on proper administration
- Monitoring for adverse effects
- Supporting patient adherence
The plozasiran FDA approval introduces new responsibilities but also new opportunities for improved patient care.
Comparison With Existing Therapies
Before the plozasiran FDA approval, treatment options for FCS were extremely limited. Traditional lipid-lowering drugs often failed to provide adequate benefit.
Advantages of Plozasiran
- Targets disease mechanism directly
- Provides profound triglyceride reduction
- Less frequent dosing
- Better tolerability in many patients
This positions plozasiran as a preferred option for eligible patients following the plozasiran FDA approval.
Economic and Healthcare System Implications
As a rare disease therapy, plozasiran represents a high-value intervention. While treatment costs may be significant, the reduction in hospitalizations and pancreatitis-related complications may offset long-term expenses.
The plozasiran FDA approval is likely to influence:
- Insurance coverage policies
- Rare disease funding frameworks
- Health technology assessments
Future Research and Development
The approval of plozasiran opens doors for further innovation in RNA-based therapeutics.
Potential future directions include:
- Expanded indications beyond FCS
- Combination therapies
- Long-term outcome studies
- Personalized lipid management strategies
The plozasiran FDA approval sets a precedent for next-generation lipid therapies.
Regulatory and Industry Impact
From a regulatory perspective, the plozasiran FDA approval reinforces the FDA’s commitment to rare disease drug development. For the pharmaceutical industry, it highlights the growing importance of RNA interference platforms.
This approval may accelerate:
- Investment in RNA-based drugs
- Faster development timelines for rare diseases
- Collaborative research initiatives
Conclusion
The FDA approval of Plozasiran marks a transformative moment in the treatment of familial chylomicronemia syndrome. By targeting ApoC-III at the molecular level, Plozasiran offers a powerful, precise, and patient-centric treatment approach that addresses the root cause of severe hypertriglyceridemia.
For patients, this means hope and improved quality of life. For clinicians and pharmacists, it provides a scientifically advanced tool for managing a previously intractable condition. For the pharmaceutical industry, it heralds a new era of RNA-based innovation.
As real-world experience grows, the true impact of Plozasiran’s FDA approval will continue to unfold—reshaping the treatment of rare diseases and setting new standards for precision medicine.

