Table of Contents
Introduction to Rystiggo Prescribing Information :
When managing generalized myasthenia gravis (GMG), a rare neuromuscular disorder, Restigo (rosanolixizumab-noli) has emerged as an unprecedented treatment option. Approved by the FDA, Restigo is a humanized IgG4 monoclonal antibody designed for patients with anti-AChR or anti-MUSK antibodies.
In this guide, we will explore the Restigo prescribing information in detail, including approved indications, dosage recommendations, warnings, side effects, and more to ensure optimal and safe use. This article is a valuable resource for healthcare professionals and patients.
What is myasthenia gravis (GMG)?
- Myasthenia gravis (MG) is a chronic autoimmune neuromuscular disorder that causes weakness in the skeletal muscles responsible for movement. When the term generalized myasthenia gravis (GMG) is used, it refers to a more widespread form of the disease, which affects many muscle groups beyond the eyes and face – including the muscles of the arms, legs, throat, and breathing.
- In MG, the body’s immune system produces antibodies that attack or block acetylcholine receptors (AChRs) at the neuromuscular junction — the point where nerve cells communicate with muscles. This disrupts normal nerve-to-muscle communication, leading to muscle weakness.
- Some patients may have antibodies against MuSK (muscle-specific kinase) or other related proteins instead of AChR.
What is Rystiggo?
Generic Name: Rozanolixizumab-noli
Brand Name: Rystiggo
Drug Class: Neonatal Fc receptor blocker
Route of Administration: Subcutaneous infusion
Approval: FDA approved in June 2023
Rystiggo is indicated for the treatment of adults with generalized myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody-positive or anti-muscle-specific kinase (MuSK) antibody-positive. Lets see Mechanism of action of rystiggo before ,Rystiggo prescribing information.
Mechanism of Action :
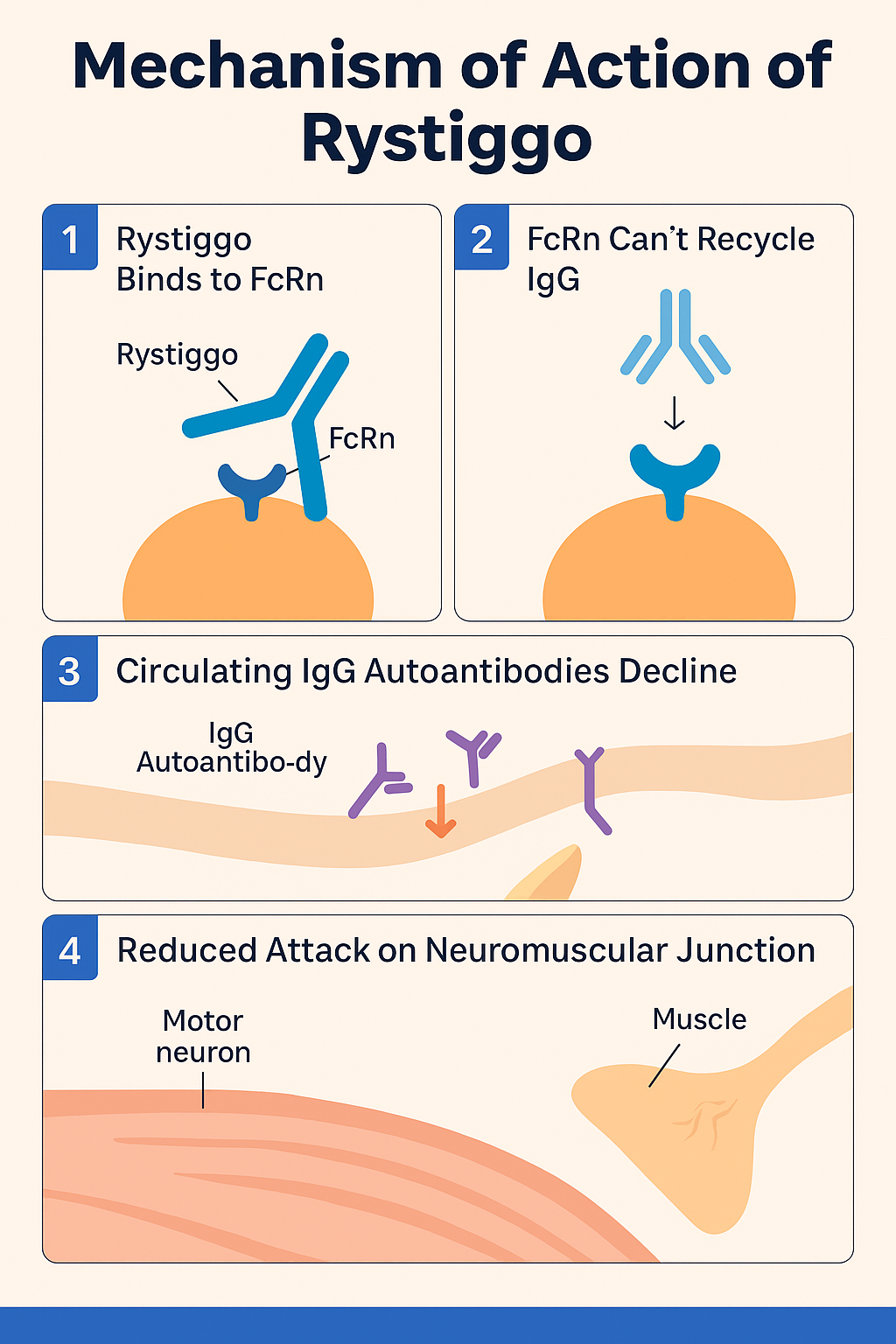
1.FcRn Function in the Body–
Normally, FcRn binds to IgG antibodies inside cells and recycles them back into the bloodstream, which prolongs the half-life of IgG antibodies, including both normal and harmful (autoimmune) ones.
2.Rystiggo Blocks FcRn–
Rystiggo binds to FcRn receptors and prevents them from recycling IgG antibodies. This leads to the destruction of IgG antibodies, including pathogenic autoantibodies involved in generalized myasthenia gravis (gMG).
3.Reduction of Pathogenic Autoantibodies –
In gMG, autoantibodies attack acetylcholine receptors or muscle-specific kinase (MuSK) at the neuromuscular junction. By reducing these circulating IgG autoantibodies, Rystiggo improves neuromuscular transmission, leading to better muscle strength and symptom relief.
Rystiggo Dosage and Administration:
Rystiggo prescribing information specifies dosing based on body weight. The drug is administered via subcutaneous infusion by a healthcare professional.
1.Recommended Dosage –
- For 40 kg to <60 kg: 420 mg once weekly for 6 weeks
- For 60 kg to <100 kg: 560 mg once weekly for 6 weeks
- For ≥100 kg: 840 mg once weekly for 6 weeks
2.Administration Instructions–
- Administer Rystiggo via subcutaneous infusion over approximately 20 to 30 minutes.
- No dilution required; provided in a single-dose vial.
- Rotate injection sites for each dose.
- Monitor patients for any infusion reactions.
💡 Note: Retreatment may be necessary based on clinical assessment.
Contraindications :
Rystiggo prescribing information includes Rystiggo contraindication in patients who :
- Known hypersensitivity to rozanolixizumab-noli or any excipients
- Ongoing serious infections
Rystiggo prescribing information Includes Warnings and Precautions :
1. Infections
Patients on Rystiggo are at increased risk for infections due to immune modulation. Monitor for signs of:
- Upper respiratory infections
- Urinary tract infections
- Sepsis (rare but serious)
2. Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, have been reported. Discontinue immediately if symptoms arise.
3. Vaccinations
Avoid live or live-attenuated vaccines during Rystiggo treatment and for at least 6 weeks after therapy ends.
4. Pregnancy and Lactation
There is limited data on the use of Rystiggo in pregnant or lactating women. Use only if clearly needed.
Adverse Reactions :
According to clinical trials and prescribing data, common side effects include:
1.Common Side Effects –
| Common Side Effects | Frequency |
|---|---|
| Headache | 45% |
| Diarrhea | 27% |
| Pyrexia (Fever) | 18% |
| Nausea | 16% |
| Infusion site reactions | 12% |
2.Serious Adverse Reactions–
- Infections (pneumonia, sepsis)
- Hypersensitivity reactions
- Neutropenia (low white blood cell count)
Drug Interactions:
There are no significant drug interactions reported for Rystiggo. However, avoid concurrent use with:
- Other immunosuppressants without proper medical supervision
- Live vaccines
Healthcare providers should evaluate individual medication profiles before initiating therapy.
Monitoring Parameters :
Patients on Rystiggo should undergo regular monitoring:
- Signs of infection
- CBC (Complete Blood Count) to track immune status
- IgG levels (if needed)
Storage and Handling :
Rystiggo prescribing information specifies the storage and handling condition for Rystiggo
- Store refrigerated at 2°C to 8°C (36°F to 46°F)
- Do not freeze or shake
- Keep vials in the original carton to protect from light
- Use immediately after removing from the refrigerator (within 4 hours)
Patient Counseling Tips :
Healthcare professionals should educate patients about:
- The purpose of Rystiggo in treating gMG
- Proper schedule adherence for dosing
- Reporting any signs of infection or allergic reactions
- Avoiding live vaccines during and after treatment
Conclusion :
Restigo offers a promising new therapy for patients with generalized myasthenia gravis who previously had limited options. By understanding the Rystiggo prescribing information included in the prescription of Restigo – including dosage, warnings and side effects – healthcare professionals can ensure safer and more effective treatment outcomes.
Frequently Asked Questions (FAQs)
1. What is Rystiggo used for?
2. How is Rystiggo administered?
3. Can I receive vaccines while on Rystiggo?
4. What should I do if I miss a dose?
5. Is Rystiggo safe during pregnancy?
5. Is Rystiggo safe during pregnancy?
6. Are there any long-term side effects?
Disclaimer: This blog post is for informational purposes only and does not constitute medical advice. Always consult a qualified healthcare professional before making any medical decisions.

